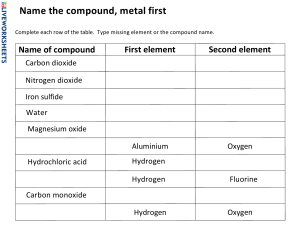
The Periodic Table 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Atomic number 1 H 1.01 2 He 4.00 Element 4 Be 9.01 3 11 Na 22.99 12 Mg 24.31 4 19 K 39.10 20 Ca 40.08 21 Sc 44.96 22 Ti 47.87 23 V 50.94 24 Cr 52.00 25 Mn 54.94 5 37 Rb 85.47 38 Sr 87.62 39 Y 88.91 40 Zr 91.22 41 Nb 92.91 42 Mo 95.96 43 Tc (98) 6 56 57 † 72 55 Ba La Cs Hf 132.91 137.33 138.91 178.49 73 Ta 180.95 74 W 183.84 7 87 Fr (223) 104 Rf (267) 105 Db (268) 106 Sg (269) 107 Bh (270) 58 Ce 140.12 59 Pr 140.91 60 Nd 144.24 61 Pm (145) 90 Th 232.04 91 Pa 231.04 92 U 238.03 93 Np (237) Relative atomic mass 89 ‡ Ac (227) † ‡ 26 Fe 55.85 27 Co 58.93 28 Ni 58.69 29 Cu 63.55 30 Zn 65.38 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F 19.00 10 Ne 20.18 13 Al 26.98 14 Si 28.09 15 P 30.97 16 S 32.07 17 Cl 35.45 18 Ar 39.95 31 Ga 69.72 32 Ge 72.63 33 As 74.92 34 Se 78.96 35 Br 79.90 36 Kr 83.90 45 48 54 50 46 44 49 51 47 52 53 In I Rh Cd Xe Sn Pd Ru Sb Ag Te 101.07 102.91 106.42 107.87 112.41 114.82 118.71 121.76 127.60 126.90 131.29 75 80 78 76 81 79 77 Re Pt Os Tl Au Ir Hg 186.21 190.23 192.22 195.08 196.97 200.59 204.38 108 Hs (269) 109 Mt (278) 110 Ds (281) 111 Rg (281) 112 Cn (285) 113 Unt (286) 82 Pb 207.2 83 Bi 208.98 84 Po (209) 85 At (210) 86 Rn (222) 114 Uug (289) 115 Uup (288) 116 Uuh (293) 117 Uus (294) 118 Uuo (294) 66 68 64 62 67 69 65 63 70 71 Dy Sm Tm Tb Lu Er Gd Ho Eu Yb 150.36 151.96 157.25 158.93 162.50 164.93 167.26 168.93 173.05 174.97 94 Pu (244) 95 Am (243) 96 Cm (247) 97 Bk (247) 98 Cf (251) 99 Es (252) 100 Fm (257) 101 Md (258) 102 No (259) 103 Lr (262) N19/4/CHEMI/HPM/ENG/TZ0/XX 3 Li 6.94 –2– 2 88 Ra (226) 18 Ionic formulae The other type of formulae you need to be able to write are those of ionic compounds. Compounds made up of positive cations and negative anions. To write these you need to know or have access to a table of ions. Table of Ions for Yr10 Chemistry Cations (metal ions) 1± H± 2± hydrogen 3± Mg2+ magnesium Al‹± aluminium Na± sodium Ca2+ calcium Fe‹± iron(III) K± potassium Ba2+ barium NH¢± ammonium Fe2+ iron(II) Ag± silver Cu2+ copper Li± lithium Zn2+ zinc Sn2+ tin Pb2+ lead Note other names, Fe"± ferrous, Fe‹± ferric, Cu"± cupric. Anions (non-metal ions) 1— 2— 3— OH— hydroxide O2- oxide PO¢‹— phosphate F— fluoride S2- sulphide N‹— nitride Cl— chloride CO£2- carbonate P‹— phosphide Br— bromide SO¢2- sulphate I— iodide SO£2- sulphite NO£— nitrate HCO£— hydrogencarbonate CH£COO— ethanoate Yr 10 booklet 2013, page 42 Name______________________________________ 3. 0.487 g sample of quinine 324g/mol is combusted and found to produce 1.321 g carbon dioxide 0.325 g water and 0.0421 g nitrogen. Determine in empirical and molecular formula (hint there is one element missing!) 4. After combustion with excess oxygen, a 12.501 g of a petroleum compound produced 38.130 g of carbon dioxide and 18.732 of water. A previous analysis determined that the compound does not contain oxygen. Establish the empirical formula of the compound 5. A hydrocarbon fuel is fully combusted with 22.000 g of oxygen to yield 23.118 g of carbon dioxide and 4.729 g of water. Find the empirical formula for the hydrocarbon. 6. 12.915 g of a biochemical substance was burned in an atmosphere of 50.123 g of oxygen. Subsequent analysis of the gaseous result yielded 18.942 g carbon dioxide, 7.749 g of water and 36.347 g of oxygen. Determine the empirical formula of the substance. 7. A 12.00 gram sample of a smelly compound was tested by combustion analysis. The products were 21.41 grams of CO2, 14.59 grams of H2O, and 17.51 grams of N2O5. Further analysis showed that oxygen was NOT present in the molecule. What is the empirical formula of the compound? 8. When a 10.0 g sample of an unknown organic acid is subjected to combustion analysis 21.2 grams of CO2 and 3.25 g of H2O are produced. What is the empirical formula of the acid? Answers: 3. C10H12NO, C20H24N2O2 4. C5H12 when I re-did the answer, I noticed that the ratio was off. The answer is not C3H7 5. CH, there is no oxygen in the compound and the amount of oxygen reacted is excess. 6. CH2O, oxygen is in excess. You can’t use it to find the amount of oxygen in the compound. 7. C3H10N 8. C4H3O2 Empirical formula and combustion analysis worksheet Page 8 of 8 3/4/18 –3– 1. 2. M10/4/CHEMI/HPM/ENG/TZ2/XX+ What is the mass, in g, of one molecule of ethane, C2H6? A. 3.0 10 23 B. 5.0 10 23 C. 30 D. 1.8 1025 6.0 mol of aluminium reacts with oxygen to form aluminium oxide. What is the amount of oxygen, in mol, needed for complete reaction? 4Al (s) + 3O2 (g) → 2Al2O3 (s) 3. A. 1.5 B. 3.0 C. 4.5 D. 6.0 Which of the following is consistent with Avogadro’s law? A. B. 4. P T V T C. Vn D. V n constant (V, n constant) constant (P, n constant) constant (P, T constant) constant (P, T constant) A sample of element X contains 69 % of 63X and 31 % of 65X. What is the relative atomic mass of X in this sample? A. 63.0 B. 63.6 C. 65.0 D. 69.0 2210-6113 Turn over