Physics 225 * Test #1 - University of St. Thomas
advertisement
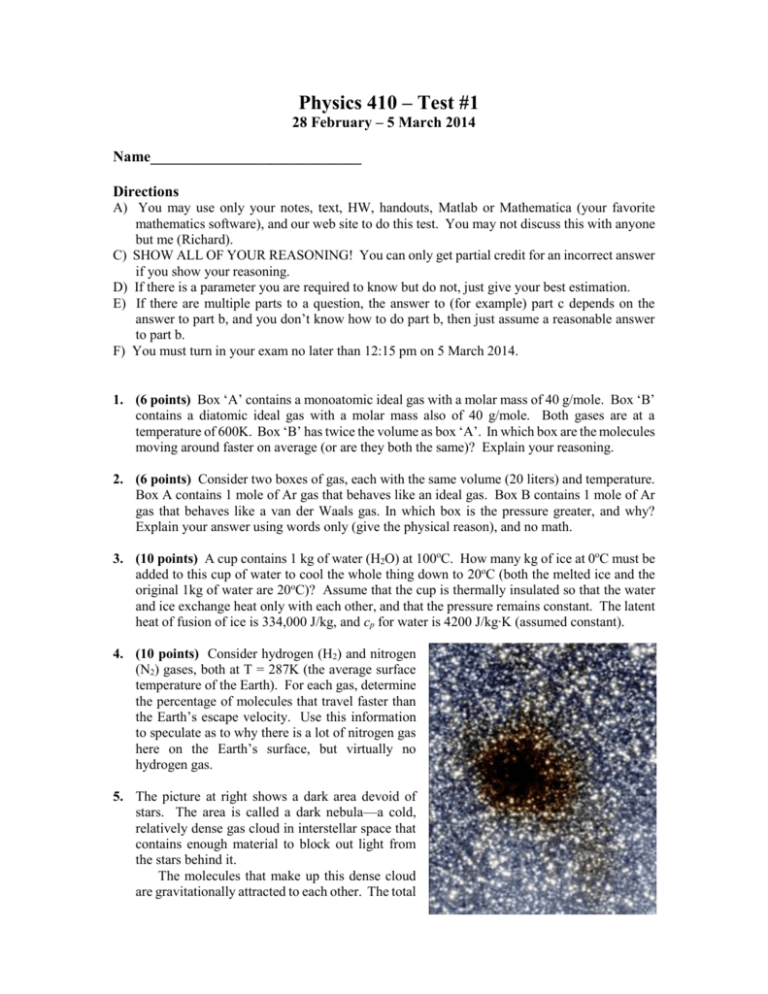
Physics 410 – Test #1 28 February – 5 March 2014 Name____________________________ Directions A) You may use only your notes, text, HW, handouts, Matlab or Mathematica (your favorite mathematics software), and our web site to do this test. You may not discuss this with anyone but me (Richard). C) SHOW ALL OF YOUR REASONING! You can only get partial credit for an incorrect answer if you show your reasoning. D) If there is a parameter you are required to know but do not, just give your best estimation. E) If there are multiple parts to a question, the answer to (for example) part c depends on the answer to part b, and you don’t know how to do part b, then just assume a reasonable answer to part b. F) You must turn in your exam no later than 12:15 pm on 5 March 2014. 1. (6 points) Box ‘A’ contains a monoatomic ideal gas with a molar mass of 40 g/mole. Box ‘B’ contains a diatomic ideal gas with a molar mass also of 40 g/mole. Both gases are at a temperature of 600K. Box ‘B’ has twice the volume as box ‘A’. In which box are the molecules moving around faster on average (or are they both the same)? Explain your reasoning. 2. (6 points) Consider two boxes of gas, each with the same volume (20 liters) and temperature. Box A contains 1 mole of Ar gas that behaves like an ideal gas. Box B contains 1 mole of Ar gas that behaves like a van der Waals gas. In which box is the pressure greater, and why? Explain your answer using words only (give the physical reason), and no math. 3. (10 points) A cup contains 1 kg of water (H2O) at 100oC. How many kg of ice at 0oC must be added to this cup of water to cool the whole thing down to 20oC (both the melted ice and the original 1kg of water are 20oC)? Assume that the cup is thermally insulated so that the water and ice exchange heat only with each other, and that the pressure remains constant. The latent heat of fusion of ice is 334,000 J/kg, and cp for water is 4200 J/kg∙K (assumed constant). 4. (10 points) Consider hydrogen (H2) and nitrogen (N2) gases, both at T = 287K (the average surface temperature of the Earth). For each gas, determine the percentage of molecules that travel faster than the Earth’s escape velocity. Use this information to speculate as to why there is a lot of nitrogen gas here on the Earth’s surface, but virtually no hydrogen gas. 5. The picture at right shows a dark area devoid of stars. The area is called a dark nebula—a cold, relatively dense gas cloud in interstellar space that contains enough material to block out light from the stars behind it. The molecules that make up this dense cloud are gravitationally attracted to each other. The total gravitational potential energy of the molecules in a spherical cloud is given by 3 𝐺(𝑁 𝑚)2 𝑃𝐸 ≈ − 5 𝑅𝑐 where 𝐺 is the gravitational force constant (6.6742910-11 m3/(kg s2)), 𝑁 is the number of molecules in the cloud, 𝑚 is the mass of one molecule, and 𝑅𝑐 is the radius of the cloud. If the total kinetic energy of the molecules in the cloud is less than the absolute value of the above potential energy function, then the cloud will gravitationally collapse and form a star/solar system. Consider a spherical dark nebula of radius 1017 m (about 10 light years) that contains 6 35 10 moles of atomic hydrogen (radius of H atom = 0.53 10-10m). a. (7 points) Determine the maximum temperature of the atomic hydrogen gas that would allow this cloud to collapse gravitationally. Now assume that the temperature of the H atoms in the nebula is ½ that calculated in part a. b. (6 points) Estimate the mean free path for a hydrogen atom in the cloud. c. (6 points) Estimate the average time between collisions for a typical hydrogen atom in the cloud. (NOTE: Of course, molecular clouds contain more than just H atoms. I oversimplified here to make the problem more doable.) 6. n moles of a diatomic ideal gas originally at a temperature T1 and P pressure 3P1 expands reversibly against a frictionless piston to twice its original volume. The temperature of the gas is varied 3P1 during the expansion so that at each instant the path in the P-V diagram is one quarter of an ellipse. Assume all (3D) degrees of freedom are accessible between these two temperatures. (NOTE: The area of an ellipse is 𝜋𝑎𝑏, where 𝑎 and 𝑏 are ½ the lengths of P1 the major and minor axes, respectively. a. (7 points) Find the final temperature T2 in terms of T1. V b. (7 points) Find the work done by the gas in terms of n, R, and T1. V1 2V1 c. (6 points) Find the quantity of heat the gas exchanges with the environment in terms of n, R, and T1. Does the system absorb or reject heat during this process? d. (6 points) Determine the change in enthalpy of this process in terms of n, R, and T1. 7. Consider the Berthelot equation of state: 𝑎𝑛2 (𝑃 + 2 ) (𝑉 − 𝑛𝑏) = 𝑛𝑅𝑇 𝑉 𝑇 Using this equation of state, derive expressions for the work done by a gas for the following two reversible processes. Evaluate all integrals. a. (6 points) isobaric (expression in terms of V1, V2, P, n, a, b) b. (7 points) isothermal (expression in terms of V1, V2, T, n, a, b) 𝜕𝑈 𝜕𝑇 𝑃 8. (10 points) Show that ( ) = 𝑛𝑐𝑃 − 𝑃 𝑉 . _______________________________________ By signing my name above, I affirm that this test represents my work only, without aid from outside sources. In all aspects of this course I perform with honor and integrity.