Bohr-Rutherford Diagrams
advertisement

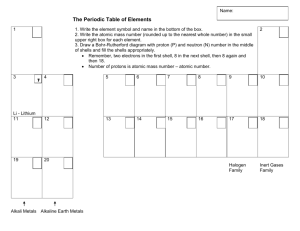
Bohr-Rutherford Diagrams Questions: 1. a. In which group are sodium, lithium and potassium? b. How many electrons are in the outer shell of sodium, lithium and potassium? 2. a. How many electrons are in the outer shell of fluorine and chlorine? b. In which group are fluorine and chlorine? 3. Examine the other groups, what general statement can be made with regards to the group number and the number of electrons in the outershell? 4. a. In which period are hydrogen and helium? b. How many shells of electrons are in the atoms of hydrogen and helium? 5. a. In which period are lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine and neon? b. How many shells of electrons do the above atoms have in their BohrRutherford diagram? 6. In general, what is the relationship between the period number and the number of shells of electrons? 7. a. How many electrons does shell #1 hold? b. How many elements are in period #1? 8. a. How many electrons does shell #2 hold? b. How many elements are in period #2? 9. What is the relationship between the maximum number of electrons in each shell and the number of elements in each period?