(CH 2 ) 4 CH 3 Hexyl
advertisement

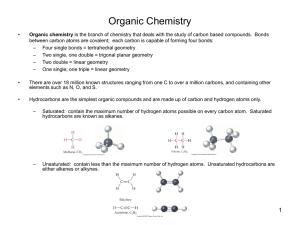
Organic Chemistry Carbon atoms and Organic Compounds Carbon can have 4 bonds. These bonds can form in chains, rings, and branching networks to form o Synthetic polymers-(man-made polymers) such as petroleum oil products- plastics o Natural polymers – (naturally occurring) macromolecules (Nucleic Acids-DNA, RNA, Carbohydrates, Proteins, and Fats). Organic Compounds consist of two things: 1. Covalent compound 2. Must have Carbon atoms (usually H too) a. C17H19NO3 – morphine (organic) b. C19H28O2 – testosterone (organic) Allotropes of Carbon 1. Diamonds 2. Graphite 3. Fullerene (discovered in mid-1980’s) a. Nearly spherical b. Most stable form is C60-buckminister fullerene c. Forms in soot when Carbon is burned with a limited amount of Oxygen 4. Nanotube (discovered in 1991) a. Hexagons that form a hollow cylinder b. 10,000 times smaller than a human hair and between 10 to 100 times stronger than steel by weight Hydrocarbons-only contain only Carbon and Hydrogen atoms Three types: (See naming organic chemicals for chemical formulas) 1. Alkanes (only single bonds) 2. Alkenes (single bonds and at least one double bond) 3. Alkynes (single bonds with at least one triple bond) Two kinds: 1. Saturated Hydrocarbon-each Carbon atom has 4 single bonds (alkanes) 2. Unsaturated Hydrocarbon-Each molecule has at least one double or triple bond (alkenes and alkynes) 1 Isomers-Are 2 molecules with the same chemical formula but have different properties. C4H10O names: 1-butanol and 2-methyl-1-propanol Naming and Uses of Organic Acids and Bases First you need to determine the functional group. Note: You can name organic compounds by their IUPAC name or Common name. 1. Alkanes: Family of saturated hydrocarbons. Cn H2n + 2 Molecules that contain only H and C that are connected by single bonds and with –ane ending. Ex. Methane: CH4 (1 Carbon and 4 Hydrogens) Has sp3 hybrid, tetrahedral structure, with bond angles of 109.5 degrees. cyclo-A prefix added to an Alkane means a ring is included in the chemical formula Others:2-14 (IUPAC Names) CH3CH3 Ethane CH3CH2CH3 Propane CH3(CH2)2CH3 Butane CH3(CH2)3CH3 Pentane CH3(CH2)4CH3 Hexane CH3(CH2)5CH3 Heptane Alkyl Group: If a H atom is removed from an alkane, a partial structure that remains is an alkyl. Alkyl groups are named by replacing the –ane ending of the parent alkane with an –yl ending. Ex. Methyl: -CH3 Others: -CH2CH3 Ethyl -CH2CH2CH3 Propyl -CH2(CH2)2CH3 Butyl -CH2(CH2)3CH3 Pentyl -CH2(CH2)4CH3 Hexyl The alkyl groups are often used to determine the common names of compounds. R represents an alkyl group 2. Alkenes: Family of unsaturated hydrocarbons with Carbon=Carbon double bonds. Cn H2n Since fewer H’s than in alkanes they are unsaturated with an –ene ending. Ex. Ethylene (IUPAC name is ethene): H2C=CH2 Ex. Propylene (IUPAC name is propene): CH3CH=CH2 Has sp2 hybrid, planar structure with bond angles of about 120 degrees. Benzene is a aromatic hydrocarbon organic ring with C6H6 3. Alkynes: Family of unsaturated hydrocarbons with CarbonCarbon triple bonds. Cn Hn alkynes use the suffix: -yne Line drawing representation would be RCCH Ex. Propyne: CH3CCH Has sp hybrid, linear structure with bond angles of 180 degrees. Ex. Acetylene (IUPAC name is ethyne): H-CC-H Used to prepare acetic acid Other Functional Groups (8 total) 1. Esters : common in plants and responsible for some distinctive flavors and scents (like perfume) O ║ C˗O Flavor of pineapple is caused by ethyl butyrate 2. Amines: Family of organic derivatives of ammonia, NH3. 2 Amines are named by adding the ending –amine to the name of the hydrocarbon from which it is derived. Organic bases contain nitrogen with unshared pair of electrons. Line drawing representation would be R-NH2 Ex. Methylamine H3C-NH2 and Ethanamine CH3CH2-NH2 Found in caffeine –coffee drinks and soft drinks 3. Alcohols: -OH Example: Ethanol (liquor) is used as a solvent for many flavorings: vanilla extract 4. Halide: -Group 17 elements 5. 6. 7. 8. Example: Freon-11 which is a refrigerant Ketones: Example-Acetone (propanone) used for nail polisher remover O ║ C Aldehyde: Example- almond extract (benzaldehyde) O ║ C˗H Ether: -O- are used in perfumes Carboxylic Acid: Family of the most useful building blocks for synthesizing other molecules. O ║ Line drawing representation would be C R OH They are named by adding the ending –oic acid to the name of the hydrocarbon from which the acid is derived. Ex. HCOOH is methanoic acid, which is also known as formic acid. Found in soap Derivatives of carboxylic acid are: O ║ C R X Acid halide (X=F, Cl, Br, or I) With ending –yl halide Ex. Acetyl chloride (from acetic acid) where the R is CH3 and the X is Cl. Ex. Acetic anhydride where both the R’s are replaced with CH 3. O O ║ ║ C C R O R Acid anhydride: With ending anhydride 3