Spectroscopic studies on tungstoheteropoly anions functionalized
advertisement

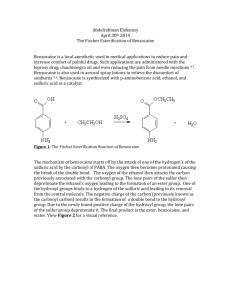
Spectroscopic studies on tungstoheteropoly anions functionalized by amino acids M. Mirzaei*, a, H. Eshtiagh-Hosseini a, M. Arefian a, F. Akbarnia a, F. Miri a, S. Edalatkar – Moghadam b and M. Shamsipur c a Department of Chemistry, Ferdowsi University of Mashhad, 91775-1436 Mashhad, Iran e-mail: mirzaeesh@um.ac.ir,bDepartment of Chemistry , Shar-e Rey Branch , Islamic Azad University,Tehran , Iran, c Department of Chemistry, Razi University, Kermanshah, Iran Fig S1. IR spectra of synthesized K6P2W18O62 1 The procedure for the synthesis of Gly-Ala.P2 (12)wasaccomplished by dissolving 0.3 g (0.06 mmol) of presynthesized P2W18O62 in acetonitrile,0.048 g (0.54 mmol)alanine in 4 ml ethanol and 4 drops of HCl (2N),then a soloution of 0.032 g (0.42 mmol)glycine in 4 ml ethanol added dropwisely to first solution. The result solution was refluxed for 4 hours in 90°C.Slow evaporation of the solvent at room temperature led toBlock blue crystals after about three months. a b Fig S2. IR spectrum (a) and HNMR (b) of Ala-Gly.P2 The procedure for the synthesis ofGly-Gln.P2 (13) was identical to that of 12, but 0.027 g (0.36 mmol) glycine in 10 ml distilled water and 0.71 g (0.48 mmol) glutamine in 4 ml H2O, added to a solution of 0.3g (0.06mmol) P2.after some days block blue crystals of 13 appeared. a 2 b c Fig S3. IR spectrum(a), 1HNMR(b) and 13CNMR of Gly-Gln.P2 Theprocedure for the synthesis of Gly-Ala.P2 (12)wasaccomplished by dissolving 0.93 g (2.84mmol) of Na2WO4 in 30 ml distilled water and 1ml H3PO4 (85%). Then 0.76 g (8.52 mmol)alanine and 1.59 g (5.52 mmol) sodium glutamate added to first solution. pH adjusted to 3 by HCl (2N)and stirred for 30 minutes. a 3 c b Fig S4. IR spectrum(a), 1HNMR(b) and 13CNMR(c) of Ala-Gln.P2 The procedure for the synthesis ofGln-Asn.P2 (14) was identical to that of 12, but 0.033 g (0.24mmol) glutamine in 3ml ethanol and 1 drop HCl (2N), added to a solution of 0.3g (0.06mmol) P2 in 9 ml acetonitrile. Then a solution of 0.033g (0.84mmol) asparagine in 3ml ethanol and 1ml HCl added to previous solution. After some days block blue crystals of 14 appeared. a 4 c b Fig S5. IR spectrum(a), 1HNMR(b) and 13CNMR(c) of Gln-Asn.P2 The procedure for the synthesis of Gly.P2 (17) accomplished by dissolving 0.06 g (0.8mmol) glycine in 2ml HCl (2N)added to solution of 0.303g (0.06mmol) P2 in 10 ml distilled water. Then stirred 3 hours at room temperature. After some months block yellow crystals of 17 appeared. 5 Fig S6. IR spectrum of Gly.P2 The procedure for the synthesis of Gly-Thr.P2 (15) was identical to that of 12, but 0.014 g (0.12 mmol) threonine in 3ml ethanol and 1 drop HCl (2N), added to a solution of 0.2g (0.04mmol) P2 in 6 ml acetonitrile. Then a solution of 0.012g (0.12mmol) glycine in 3ml ethanol and 1ml HCl added to previous solution. After 20 days greenish crystals of 15 appeared. a b c Fig S7. IR spectrum(a), 1HNMR(b) and 13CNMR(c) of Gly-Thr.P2 6 The procedure for the synthesis of Ala-Asp.P2 (11) was identical to that of 12, but 0.018 g (0.2 mmol) alanine and 0.017g (0.12mmol) aspartic acid in 4 ml HCl (2N), added to a solution of 0.102g (0.02mmol) P2 in 6 ml acetonitrile and refluxed for 4 hours in 90°C. After a few monthsyellowish crystalsappeared. a b c Fig S8. IR spectrum(a), 1HNMR(b) and 13CNMR(c) of ASp-Ala.P2 7 a b Fig S9. IR spectra of P5 (a) and IR spectra in 600-1200 cm-1 (b) 8 The procedure for the synthesis of Asp-Ala.P5 (1) wasaccomplished by dissolving presynthesized [Na(H2O)P5W30O110]14- anion (P5)in 10 ml distilled water,0.041 g (0.46 mmol)alanine in 4 ml ethanol and 4 drops of HCl (2N)added to first solution, then a solution of 0.061g (0.45 mmol)aspartic acid in 5 ml ethanol added dropwisely to above solution. The result solution was refluxed for 3 hours in 90°C. Slow evaporation of the solvent at room temperature led to block colorless crystals after 45 days. a b c Fig S10. IR spectra (a), 1HNMR (b) and 13CNMR of Asp-Ala.P5 9 The procedure for the synthesis of Gly-Ala.P5 (1) was accomplished by dissolving 0.3g (0.03 mmol)P5 in 10 ml distilled water, 0.018 g (0.02 mmol) glycine and 0.022g (0.02 mmol) alanine were mixed with each other, pH adjusted to 3 by HCl (2N) and then refluxed for 3 hours in 90°C.14 days later block colorless crystals of 2 appeared. a b c Fig S11. IR spectra (a), 1HNMR (b) and 13CNMR of Gly-Ala.P5 10 The procedure for the synthesis of Gly-Gln.P5 (3) was identical to that of 1 but, 0.015 g (0.2 mmol) glycine in 5ml ethanol and 1 drop HCl (2N), added to a solution of 0.1g (0.01mmol) P5 in 10 ml distilled water. Then a solution of 0.016g (0.11mmol) glutamine in 3ml ethanol and 1ml HCl added to previous solution and refluxed for 3 hours in 90°C.After some months block colorless crystals of 3 appeared. a b Fig S12. IR spectra (a), 1HNMR (b) of Gly-Gln.P5 11 The procedure for the synthesis of Asn-Gln.P5 (4) was identical to that of 1 but, 0.051 g (0.34 mmol) glutamine in 2ml H2O added dropwisely to a solution of 0.2g (0.02mmol) P5 in 5 ml distilled water. Then a solution of 0.045g (0.34mmol) asparagine in 2ml H2O and 1ml HCl added to previous solution and refluxed for 3 hours in 90°C. After 2 months block colorless crystals of 3 appeared. a b Fig S13. IR spectra (a), 1HNMR (b) of Asn-Gln.P5 12 Meth-Cys.P5 (5) was prepared by mixing 3 solutions as follows; 0.008g (0.5mmol) methionine in 2 ml H2O, 0.019g (0.15mmol) cysteine in 5 ml H2O and 1ml HCl (2N) and 0.2g (0.02mmol)P5 in 5 ml H2O. Then refluxed as before.2 weeks later needle like yellow crystals appeared. a b Fig S14. IR spectra (a), 1HNMR (b) of Meth-Cys.P5 13 Ala-Leu.P5 (6) was prepared exactly as 5 but, 0.21g (0.16mmol) leucine in 4 ml H2O, 0.014g (0.01mmol) alanine in 2 ml H2O and 1ml HCl (2N) and 0.2g (0.02mmol)P5 in 5 ml H2O.pH adjusted to3 by HCl (2N). Then refluxed as before. 3 months later block colorless crystals appeared. a b Fig S15. IR spectra (a), 1HNMR (b) of Ala-Leu.P5 14 To prepare Arg-Leu.P5 (7) 0.021g (0.16 mmol) leucine in 2ml H2O added dropwisely to a solution of 0.2g (0.02mmol) P5 in 5 ml distilled water. Then a solution of 0.028g (0.16mmol) arginine in 2ml H2O and 1ml HCl(2N) added to previous solution and refluxed for 3 hours in 90°C. a b Fig S16. IR spectra (a), 1HNMR (b) of Arg-Leu.P5 15 The procedure to synthesize Ala-Thr.P5 (8) was identical to that of 5 but, 0.028g (0.31mmol) alanine in 2ml H2O, 0.038g (0.31mmol) threonine in 2 ml H2O and 0.2g (0.02mmol)P5 in 5 ml H2O were mixed. pH adjusted to 3 by HCl (2N). Then refluxed as before. After a few months block colorless crystals appeared. a b c Fig S17. IR spectra (a), 1HNMR (b) and 13CNMR of Ala-Thr.P5 16 To synthesize procedures for Cys.P5 (9) and Leu.P5 (10) are just the same except for the amount of cysteine and leucine; 0.017g (0.14mmol) cysteine for 9 and 0.036g (0.27mmol) leucine for 10, dissolved in 2 ml H2O, then refluxed 3 hours in 90°C. About 2 month later needle like yellow crystals appeared for both of them. a b Fig S18. IR spectra (a), 1HNMR (b) of Cys.P5 17 a b Fig S19. IR spectra (a), 1HNMR (b) of Leu.P5 18