worksheet on elements and the periodic table
advertisement

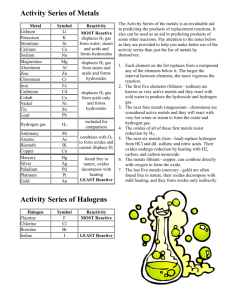
Worksheet 7.3 WORKSHEET ON ELEMENTS AND THE PERIODIC TABLE One of the simplest classifications to make is the distinction between metals and non-metals. Most of the known elements are metals. Only 22 are non-metals and of these, 11 are gases at room temperature. A few elements are hard to classify as metal or non-metal because they have some properties of each. These elements are called semi-metals, metalloids or semiconductors. 1 Use your textbook to fill in the table below, summarising the typical physical properties of metals and non-metals. (The obvious one has been done!) METALS good conductors of heat and electricity NON-METALS poor conductors As well as physical differences, there are chemical differences between metals and non-metals. A very important difference is in the acid - base behaviour of their oxides. 2 Metal oxides are ………………………… and non-metal oxides are ………………...................... Note that a few oxides are neutral (e.g. water and carbon monoxide). 3 A few oxides behave as both acids and bases. These oxides are called ………………………….. An example of this type of oxide is .................................................….. THE PERIODIC TABLE The Periodic Table shows the elements arranged in order of increasing Atomic Number. ROWS are called PERIODS : COLUMNS are called GROUPS. Groups contain similar elements and there are 8 main groups. Across a period there is a gradual change in the nature and properties of the elements. 4 On the blank grid of the Periodic Table, mark in the line which divides the metals from the nonmetals. Also on the blank grid, show where the following are:a the Alkali Metals d the Noble Gases b the Halogens e the most reactive metal c the Transition Metals f the most reactive non-metal Page 1 of 2 Worksheet 7.3 5 Which metal is a liquid at room temperature? …………………………………….…… 6 Which non-metal is a liquid at room temperature? ………………………………….……… 7 Which non-metal element is unusual in the sense that one of the forms in which it exists will conduct electricity well? ……………………….………………… 8 Name two semi-metals (metalloids or semiconductors). ………………………….……………… 9 Write down the names and symbols of three Alkali metals and give two physical similarities and two chemical similarities exhibited by this Group. NAME SYMBOL PHYSICAL SIMILARITY CHEMICAL SIMILARITY 1) 1) 2) 2) 10 Write down the names and symbols of three Halogens and give two physical and two chemical similarities shown in this Group. NAME SYMBOL PHYSICAL SIMILARITY CHEMICAL SIMILARITY 1) 1) 2) 2) 11 Give three properties which are characteristic of the Transition Metals, but not of main group metals. ....………………………………...………………………………………………………………… ....…………………..…………………….………………………………………………………… 12 What is the main characteristic of the Noble Gases? Page 2 of 2 ………….………………………………