Document
advertisement

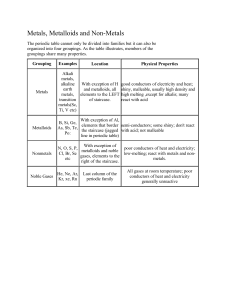
Bonds Ionic – •Bond formed due to a difference in charge – Loss or gain of an electron •Usually forms between a metal and a nonmetal Bonds Covalent – •Bond formed from two atoms sharing an electron •Commonly found in “organic” compounds (with H, C, and O, among others) Dmitri Mendeleev • Order elements by atomic mass 1834 - 1907 • Periodic Law – When the elements are arranged in order of increasing relative mass, certain sets of properties recur periodically • used pattern to predict properties of undiscovered elements Periodic Table Pattern nm H2O a/b H 1 H2 m Li2O m/nm BeOnm B2O3 nm CO2 nm N2O5 nm O2 nm OF2 Li b Be a/b B a C a N a O F 7 LiH 9 BeH2 11 ( BH3)n 12 CH4 14 NH3 16 H2O 19 HF left right across a row (period) increasing atomic mass (not relative mass); organized based on their activity and properties Periodic Table Pattern nm H2O a/b H 1 H2 m Li2O m/nm BeOnm B2O3 nm CO2 nm N2O5 nm O2 nm OF2 Li b Be a/b B a C a N a O F 7 LiH 9 BeH2 11 ( BH3)n 12 CH4 14 NH3 16 H2O 19 HF m Na2O m MgO m Al2O3 nm/m SiO2nm P4O10nm SO3 nm Cl2O7 Na b Mg b Al a/b Si a P a S a Cl a 23 NaH24 MgH2 27 (AlH3) 28 SiH4 31 PH3 32 H2S 35.5 HCl up down column; similar properties; how the elements react with H and O • some gaps were left in table for undiscovered elements; properties of these elements were predicted. PERIODIC TABLE Non-metals Metals Metalloids 7 PROPERTIES OF METALS & NON-METALS Metals Non-metals • Mostly solid • Can be solid, liquid or gas • Have shiny appearance • Have dull appearance • Good conductors of heat & electricity • Poor conductors of heat & electricity • Malleable & ductile • Brittle (if solid) • Lose electrons • Gain or share electrons 8 Metals Non-metals METALLOIDS Metalloids are elements that possess some properties of metals and some of non-metals. The most important metalloids are silicon (Si) and germanium (Ge) which are used extensively in computer chips. 10 Metalloids Properties of Silicon shiny conducts electricity brittle PERIODIC TABLE Metallic Metallic character character decreases increases going down acrossaagroup period. Least metallic Most metallic element elements F Cs Fr 12 PERIODIC TABLE Seven elements exist as diatomic molecules. All others exist as monatomic (single atom). 13 PERIODS & GROUPS The Elements periodic in the table same is composed family have of periods similar (rows) properties, and and areorcommonly referred to by their traditional groups families (columns). names. 14 PERIODS & GROUPS Group The group Elements Alkali Noble Halogens gases metals 2 elements in are ofare groups metals the areun-reactive most soft are 1-2 inmetals called reactive between and gases 13-18 alkaline-earth that nonmetals, the are that are main very referred aregroup commonly reactive. and metals. to occur as main-group used in These elements nature in metals light are only or bulbs. called are as representative compounds. less transition reactivewith than metals. groups. alkali metals. They often react explosively other elements. 15 Pick a group • Alkali metals • Alkaline earth metals • Transition Metals • Metalloids • Halogens • Noble Gasses • Name of Group • Elements in the group • Properties of the elements in the group • Bonds usually formed • Reaction to fire