Dye removal from aqueous solutions by silica microparticles
advertisement

Dye removal from aqueous solutions by silica microparticles prepared in supercritical CO2 Ersin Başaran, Tuğba Alp, Özer Gök, Adnan Özcan and A. Safa Özcan Anadolu University, Faculty of Science, Department of Chemistry, Yunusemre Campus, 26470, Eskisehir, Turkey The contamination of the industrial wastewaters with synthetic dyes which are extensively used in several industries such as textile, paper, automotive and dye production have caused serious environmental problems. The dye containing wastewaters can create hazardous effects on the living organisms because of carcinogenic, mutagenic, dermatitis and toxic [1]. The disposal of these wastewaters is a very important issue. Several methods are available to remove dye effluents from the wastewaters. In this manner there is a growing need to prepare new efficient materials in environmentally friendly processes. Supercritical fluids have been proposed as eco-friendly alternatives to traditional processes in different industrial areas. Especially, CO2 is generally used as a supercritical fluid because of its cheapness, non-toxicity, volatility and environmental impact at low critical parameters in this technology. Supercritical CO2 has utilized preparation of silica microparticles as on sphere shaped, controlled drug release agent, catalysis, column packing materials [2]. In addition to these, supercritical fluid technologies have made possible to control the size distribution of microparticles. The resulting microparticles can be used as an adsorbent for the removal of pollutants from wastewaters. In this study, silica microparticles prepared in supercritical CO2 were used to remove Acid Blue 260 (AB260) dye from aqueous solutions as a function of pH and time. After removal process, the functional groups of dye-loaded silica microparticles were determined by FTIR analysis. SEM-EDX analysis and zeta potential measurements were also performed to determine the characterization of microparticles. The results indicated that silica particles obtained in this medium were in spherical shaped. The optimum conditions for the removal of AB260 were also determined in success at pH=1.5 and 25 min. Acknowledgement: The Authors would like to acknowledge to Anadolu University Scientific Research Projects (no 1306F256 and 1409F388) for financial support. References: [1] A. Bhatnagar, A.K. Jain J. Colloid Interface Sci., 2005, 281, 49-55. [2] Alnaief, M., Smirnova, I., J. of Supercritical Fluids, 2011, 55, 1118-1123.

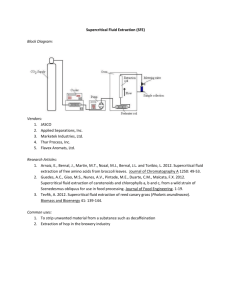





![Heat transfer mechanisms Nucleation at high subcritical pressures [1]](http://s3.studylib.net/store/data/006613018_1-484ac98340bdf87d83d3defecfde6c98-300x300.png)

