Solvothermal Synthesis of Mesoporous TiO2 Microspheres with
advertisement
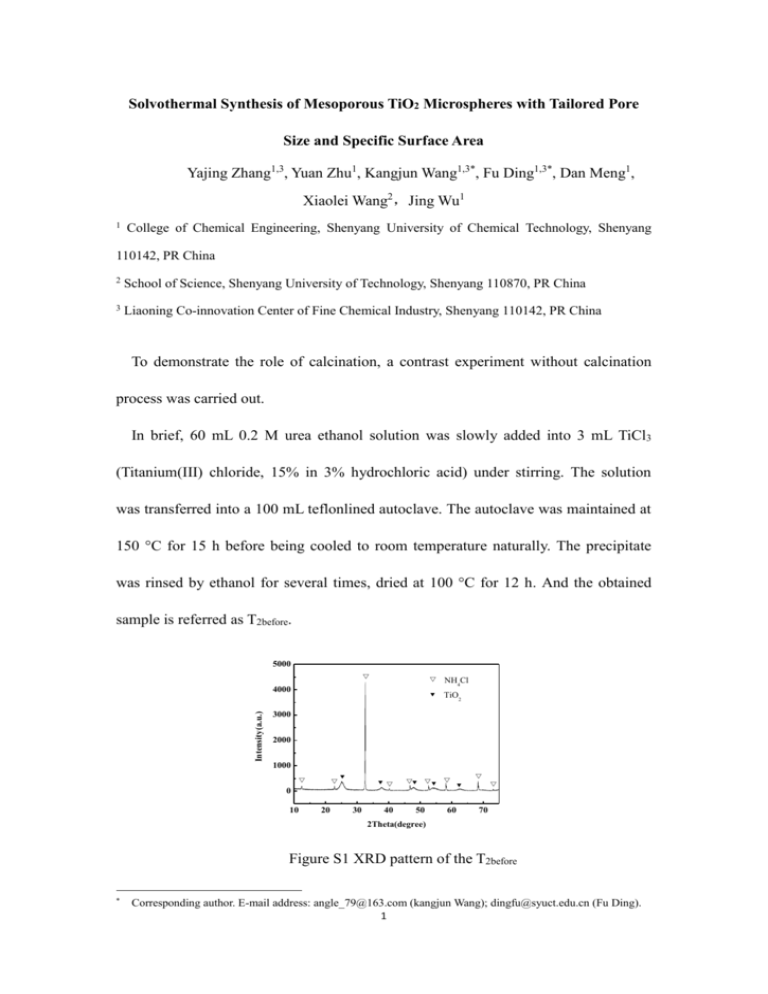
Solvothermal Synthesis of Mesoporous TiO2 Microspheres with Tailored Pore Size and Specific Surface Area Yajing Zhang1,3, Yuan Zhu1, Kangjun Wang1,3*, Fu Ding1,3*, Dan Meng1, Xiaolei Wang2,Jing Wu1 1 College of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang 110142, PR China 2 School of Science, Shenyang University of Technology, Shenyang 110870, PR China 3 Liaoning Co-innovation Center of Fine Chemical Industry, Shenyang 110142, PR China To demonstrate the role of calcination, a contrast experiment without calcination process was carried out. In brief, 60 mL 0.2 M urea ethanol solution was slowly added into 3 mL TiCl3 (Titanium(III) chloride, 15% in 3% hydrochloric acid) under stirring. The solution was transferred into a 100 mL teflonlined autoclave. The autoclave was maintained at 150 °C for 15 h before being cooled to room temperature naturally. The precipitate was rinsed by ethanol for several times, dried at 100 °C for 12 h. And the obtained sample is referred as T2before. 5000 Intensity(a.u.) 4000 NH4Cl TiO2 3000 2000 1000 0 10 20 30 40 50 60 70 2Theta(degree) Figure S1 XRD pattern of the T2before * Corresponding author. E-mail address: angle_79@163.com (kangjun Wang); dingfu@syuct.edu.cn (Fu Ding). 1 Figure S1 shows the XRD pattern of the sample without calcination process (T2before). The diffraction peaks appear at 25.17o, 38.23o, 48.09o, 55.05o, 62.83o can be indexed to TiO2 phase (Anatase, JCPDS 21-1272). And the diffraction peaks appear at 12.30o, 22.94o, 32.60o, 40.25o, 46.81o, 58.18o, 68.42o, and 73.16o can be indexed to NH4Cl phase (JCPDS 07-0007). Table 1 Specific surface area, pore volume and pore size of the samples before and after calcination Sample SBET/ (m2g-1) V/ (cm3g-1) Dpore/ (nm) T2 before 71.9 0.10 3.8 T2 106.3 0.29 7.8 2











