Supplementary Online Material
advertisement
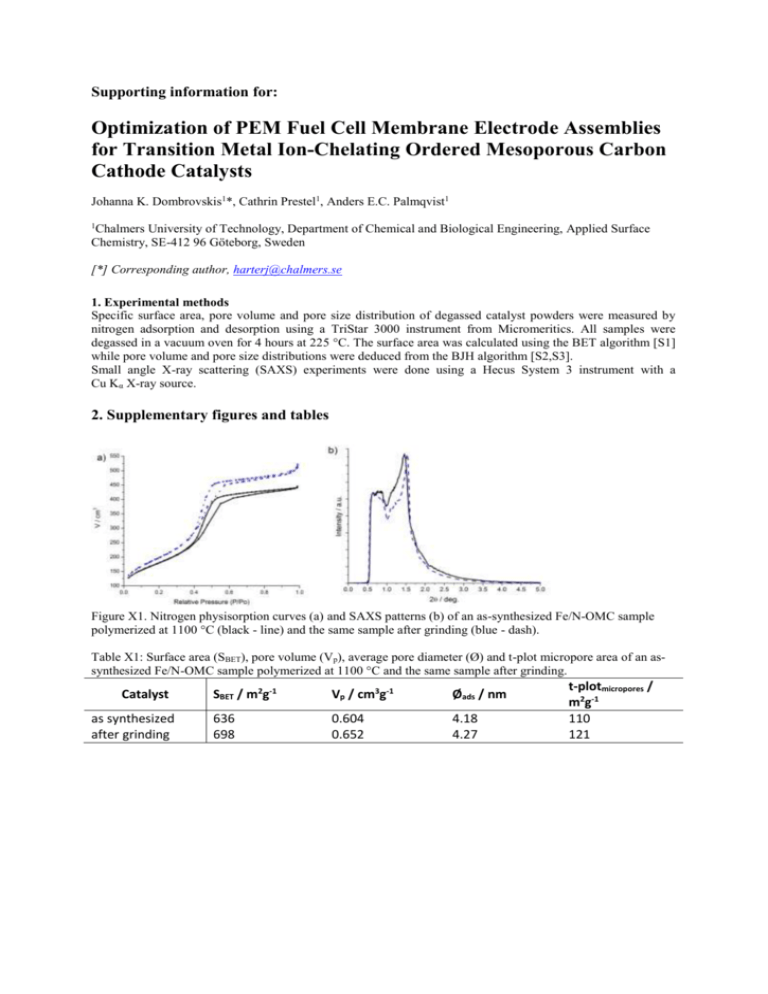
Supporting information for: Optimization of PEM Fuel Cell Membrane Electrode Assemblies for Transition Metal Ion-Chelating Ordered Mesoporous Carbon Cathode Catalysts Johanna K. Dombrovskis1*, Cathrin Prestel1, Anders E.C. Palmqvist1 1 Chalmers University of Technology, Department of Chemical and Biological Engineering, Applied Surface Chemistry, SE-412 96 Göteborg, Sweden [*] Corresponding author, harterj@chalmers.se 1. Experimental methods Specific surface area, pore volume and pore size distribution of degassed catalyst powders were measured by nitrogen adsorption and desorption using a TriStar 3000 instrument from Micromeritics. All samples were degassed in a vacuum oven for 4 hours at 225 °C. The surface area was calculated using the BET algorithm [S1] while pore volume and pore size distributions were deduced from the BJH algorithm [S2,S3]. Small angle X-ray scattering (SAXS) experiments were done using a Hecus System 3 instrument with a Cu Kα X-ray source. 2. Supplementary figures and tables Figure X1. Nitrogen physisorption curves (a) and SAXS patterns (b) of an as-synthesized Fe/N-OMC sample polymerized at 1100 °C (black - line) and the same sample after grinding (blue - dash). Table X1: Surface area (SBET), pore volume (Vp), average pore diameter (Ø) and t-plot micropore area of an assynthesized Fe/N-OMC sample polymerized at 1100 °C and the same sample after grinding. Catalyst as synthesized after grinding SBET / m2g-1 Vp / cm3g-1 Øads / nm 636 698 0.604 0.652 4.18 4.27 t-plotmicropores / m2g-1 110 121 Figure X2. Nyquist plot of the electrochemical impedance spectroscopy (EIS) measurements at room temperature and 0.6 V vs. ERHE using an optimized cathode when freshly prepared (black – filled square) and after 8 h of use (black – empty square) as well as a cathode prepared from an extensively mixed Nafion catalyst ink when freshly prepared (red – filled square) and after 8 h of use (red – empty square). 3. Supplementary references [S1] [S2] [S3] Brunauer, S.; Emmett, P.H.; Teller, E. J. Am. Chem. Soc. 1938, 60, 309-319. Barrett, E.P.; Joyner, L.G.; Halenda, P.P. J. Am. Chem. Soc.1951, 73, 373-380. Joyner, L.G.; Barrett, E.P.; Skold, R. J.Am. Chem. Soc. 1951, 73, 3155-3158.