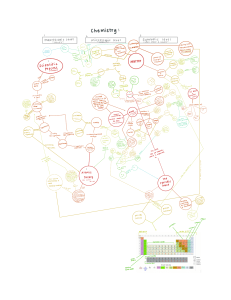
Teachers Guide Chapter 15, Section 1 1. A physical change of matter is a change that is reversible, and does not result in a new substance being formed. A chemical change is one where substance is completely changed into another substance. They are not easily reversed. 2. A 3. The Periodic Table is arranged by how elements combine with other elements, and by increasing atomic number. 4. Periods, groups 5. Metals, nonmetals, and metalloids 6. The atomic mass unit of an element is the average of all the atomic isotopes of that element. 7. 8. The alkali metals. 9. The Noble gases are called inert because they almost always are found in their pure form and do not form chemical bonds with other elements. 10. Energy levels