tpj12889-sup-0013-Legends
advertisement

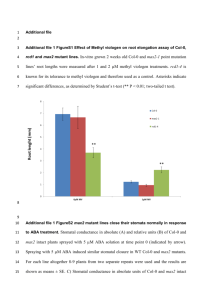
Supporting Information Legends Figure S1. Amino acid sequence comparison of SDP-related proteins in diverse plant species. The alignment was made using the ClustalW2 program. Gaps in the sequences are indicated by dashes. The position of S1 RNA-binding domain is indicated by a thick line. Accession numbers of SDP-related proteins are as follow; A. thaliana (At1g12800), Brassica rapa (CDX98043), Jatropha curcas (KDP25468), Vitis vinifera (XP00227693), Glycine max (XP003532406), Oryza sativa (NP001045672), Triticum aestivum (AEY94461), and Zea mays (AFW69797). Figure S2. Confirmation of the mutant, complementation lines, and overepxression plants. Total RNAs were extractedfrom 4-week-old Col-0, sdpmutant, complementation lines (Com1, Com2, and Com3), and overexpression lines (OX1, OX2, and OX3), and transcriptlevels of SDP were determined by RT-PCR. Figure S3. Seed yield of the wild-type,sdpmutant, and complementationlines. (a) Photographs showthe seeds of Col-0, sdpmutant, and complementation lines (Com1and Com2). Bar = 1 mm. (b) Photographs showthe siliques of Col-0, sdpmutant, and complementation lines (Com1and Com2). Bar = 1 cm. (c) Relative seed weight of Col-0, sdpmutant, and complementation lines (Com1and Com2). Figure S4. Germination and seedling growth of overexpression plants. (a) Germination rates of Col-0and overexpression plants were measured at the indicated days. (b) Seedling growth of the plants on MS medium 10 days after germination (DAG). Bar = 1 cm. (c) Growth of the plants at 30 DAG. Bar = 1 cm. Figure S5. Processing of chloroplast rRNAs is impaired in the sdpmutant. Total RNAs extracted from 4-week-old Col-0, sdpmutant, and complementation lines (Com1 and Com2) wereseparated ona1.2% formaldehyde agarose gel, and the levels of the processed products were determined by Northern blot analysisusing the probe corresponding to the sequence between 23S and 4.5S rRNAs, represented by a thick linebelow thegene.The relative intensities of the rRNA bandin the sdp mutant and complementation linewere calculatedby comparison with the band intensity in the Col-0 plant. The U6 snRNA was used as a loading control. Figure S6. Purification of the recombinant proteins. The recombinant TRX-SDPfusion proteins were expressed in E. coli, and purified using the TRX-tag. The purified proteins wereseparated via SDS-PAGE; M, molecular weight marker. Figure S7. Binding of SDP to chloroplast rRNAs. The recombinant TRX-SDP fusion proteins were incubated with the 32P-labeled rRNAs, and the mixtures were separated on native 6% acrylamide gel for 16S, 5S, and 4.5S rRNA and on 1.2% agarose gel for 23S rRNA, and the binding products were detected by a phosphorimager. Figure S8. Western blot analysis of several chloroplast-encoded proteins.Total proteins (20 μg) extracted from each sample were separated on SDS-12% PAGE, and the proteins were detected using antibody specific to each protein. Col-0/2 indicates that half amount of total protein (10 μg) was loaded in the lane.The relative intensities of each band in the sdp mutant and complementation linewere calculated by comparison with the band intensity in the Col-0 plant. Figure S9. RNA-binding ability of SDP. The recombinant TRX-SDP fusion proteins (0 to100 pmol) were incubated with 32P-labeled RNA derived from the pET22 vector, the mixtures were separated on a native 6% acrylamide gel, and the binding products were detected by a phosphorimager. Figure S10. Prediction of structural disorderness of SDP protein. The entire amino acid sequence of SDP was subjected to the prediction program DISOPRED2 (http://bioinf.cs.ucl.ac.uk/disopred) which predicts the disorderness of a peptide. Table S1. Gene-specific primer pairs used in RT-PCR and real-time RT-PCR experiments. Data S1. List of chloroplast genes and their expression levels in the sdp mutant compared with the wild-type plant.