Patho Ch17 (partial) - Esophagus through Stomach - pp753
advertisement

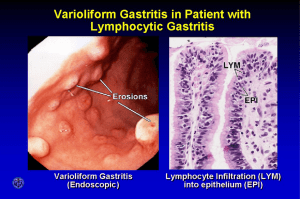
Patho Ch17 (partial) - Esophagus through Stomach - pp753-777 Esophagus Esophageal Obstruction o Structural obstructions (mechanical block) (1) Esophageal stenosis: fibrous thickening of submucosa caused by GERD (2) Esophageal mucosal webs: ledge-like protrusions of mucosa > non-progressive dysphagia (3) Esophageal rings (Schatzski rings): similar to webs, but are circumferential, thicker, and include mucosa,submucosa, and sometimes hypertrophic muscularis propria (4) Cancer: progressive dysphagia, begins with inability to swallow solids and progresses to liquids o Functional obstruction (disruption of peristalsis) (1) Nutcracker esophagus: high-amplitude contractions of distal esophagus due to loss of coordination between inner and outer SM layers (2) Diffuse esophageal spasm: repetitive simultaneous contractions of distal esophageal SM (3) Lower esophageal sphincter dysfunction: high resting pressure or incomplete relaxation, common in patients with above 2 pathologies. Can result in development of small diverticulae, especially above the lower esophageal sphincter, that can accumulate food (ex. Zenker diverticulae in >50yo) Achalasia o (1) Incomplete lower esophageal sphincter relaxation, (2) increased LES tone, and (3) aperistalsis of the esophagus o Symptoms: dysphagia for solids/foods, difficulty belching, and chest pain o Primary achalasia: distal esophageal inhibitory neuronal (ganglion cell) degeneration > inability to relax o Secondary achalasia: Chagas disease (Trypanosoma cruzi infection) causes destruction of myenteric plexus > failure of peristalsis > esophageal dilation o Rx: laparoscopic myotomy and pneumatic balloon dilaiton, or botox injection Esophagitis o Lacerations 10% of upper GI bleeding due to esophageal lacerations Mallory-Weiss tears = longitudinal tears near the gastroesophageal junction, resulting from severe vomiting secondary to alcohol intoxication. Heal on their own. Boerhaave syndrome = rare transmural tearing and rupture of distal esophagus. Requires surgery. o Chemical and Infectious Esophagitis Stratified squamous mucosa can be damaged by alcohol, acids/bases, hot fluids, and heavy smoking Pill-induced esophagitis = pills lodge and dissolve in esophagus Infections rare, but usually caused by herpes simplex virus, cytomeagalovirus, or fungal organisms Morphology: dense infiltrates of neutrophils, except in chemical damage which results in necrosis o Reflux Esophagitis Stratified squamous epithelium is resistant to abrasion but sensitive to acid LES, mucus, and bicarbonate help protect the esophagus from reflux Reflux of gastric contents into lower esophagus is the most common cause of esophagitis Pathogenesis: transient LES relaxation mediated by vagal pathways triggered by gastric distention, gas or food, pharyngeal stimulation, stress, or increased intraabdominal pressure (coughing, straining, or bending). Alcohol and tobacco use, obesity, CNS depressants, pregnancy, hiatal hernia, delayed gastric emptying, and increased gastric volume can also contribute to GERD. Morphology: mild simple hyperemia (redness), up to eosinophil/neutrophil recruitment and basal zone hyperplasia in severe cases Clinical features: GERD most common in >40yo, resulting in heartburn, dysphagia, and regurgitation of sour-tasting gastric contents. Chronic GERD can result in chest pain that can be mistaken for heart attacks. Complications: ulceration, hematemesis, melena, stricture development, and Barrett esophagus Rx: Proton pump inhibitors (have replaced H2 histamine receptor antagonists) to reduce gastric acidity o Esosinophilic Esophagitis Symptoms: food impactation and dysphagia (adults), feeding intolerance or GERD-like (children) Morphology: large number of intraepithelial eosinophils Acid reflux is not prominent and high doses of proton pump inhibitors usually don't work Rx: dietary restrictions to prevent exposure to allergens or corticosteroids o Esophageal Varices Pathogensis: portal hypertension > development of collateral channels where portal and caval systems communicate > esophageal varices o o Morphology: tortuous dilated veins within the submucosa of the distal esophagus/proximal stomach Clinical: present in nearly 1/2 patients w/ cirrhosis, 25-40% go on to develop bleeding. Variceal hemorrhage is an emergency that must be treated by inducing splanchnic vasoconstriction, injection of thrombotic agents, balloon tamponade, or variceal ligation. 30% mortality rate. 50% that survive have recurrent bleeds within a year. Barrett Esophagus Complication of chronic GERD that is characterized by intestinal metaplasia within the esophageal squamous mucosa Confers an increased risk of esophageal adenocarcinoma (however, most do not develop tumors) Morphology: one or several patches of red, velvety mucosa extending upward from the gastroesophageal junction. Risk of dysplasia correlates with length of esophagus affected. Microscopically, columnar mucosa is replaced by squamous epithelium with goblet cells. Clinical features: identified through endoscopy and biopsy, usually prompted by GERD symptoms Rx: periodic endoscopy to monitor, or surgical resection if carcinoma present. Esophageal Tumors Adenocarcinoma Most arise from Barrett esophagus Risk: tobacco use, exposure to radiation, obesity; Risk reduced: fruits and vegetables Pathogenesis: from Barrett esophagus via stepwise acquisition of genetic and epigenetic changes Morphology: usually in distal 1/3, initially appear as flat/raised patches in otherwise intact mucosa. Tumors may infiltrate in large masses or may infiltrate diffusely or ulcerate and invade deeply. Barrett esophagus is usually present adjacent to the tumor. Clinical features: commonly present with pain or difficulty in swallowing, progressive weight loss, hematemesis, chest pain, or vomiting. By the time symptoms appear, the tumor has spread to submucosal lymphatics. Squamous Cell Carcinoma Risk: alcohol and tobacco, poverty, caustic esophageal injury, achalasia, tylosis, Plummer-Vinson syndrome, diets low in fruits/vegetables, frequent consumption of very hot beverages Pathogenesis: linked to alcohol and tobacco and other mutagenic compounds. HPV has also been implicated. Morphology: 1/2 occur in the middle 1/3 of the esophagus. Begins as an in situ lesion ("squamous dysplasia") that appear as small, gray-white, plaque-like thickenings. Grow into the lumen, ulcerate, or spread into the wall and cause thickening. Symptomatic tumors are generally very large at diagnosis and have invaded the esophageal wall and lymphatic network. Clinical features: dysphagia, odynophagia (pain), or obstruction. Prominent weight loss and debilitation, hemorrhage and sepsis. Iron deficiency often present. Often, first symptoms caused by inspiration of food via trachoesophageal fistula. Stomach Gastropathy and Acute Gastritis o Gastritis: mucosal inflammatory process o Acute gastritis: lesion w/ neutrophils present o Gastropathy: if inflammatory cells are rare or absent o Risks: NSAIDs, alcohol, bile, and stress induced injury o Pathogenesis: mucus and bicarbonate secretion protects epithelium of stomach. Mucus/bicarb secreting cells are replaced every 3-7 days. Tight junction between epithelial cells prevents leakage into lamina propria. Mucosal vasculature delivers O2 and nutrients while washing away acid that has back-diffused into lamina propria. Gastropathy/gastritis can occur when any of these protective mechanisms fail. NSAIDs inhibit COX synthesis of prostaglandins, which stimulate all of the above defense mechanisms. H. pylori infection inhibiting bicarbonate transporters via ammonium ions. Reduced mucin and bicarbonate in order adults Decreased O2 delivery at higher altitudes explains increased incidence of acute gastritis Direct cellular damage via chemical ingestion or alcohol consumptions/NSAIDs/radiation/chemotherapy o Morphology: lamina propria shows only moderate edema and slight vascular congestion. Surface epithelium is intact, but secretory cell hyperplasia and epithelial proliferation are typically present. Presence of neutrophils above basement membrane indicates active inflammation. With severe mucosal damage, erosions and hemorrhage develop. Deep erosion may lead to ulcers. Concurrent erosion and hemorrhage = "acute erosive hemorrhagic gastritis". o Clinical features: presentation varies by etiology. NSAID-induced may be asymptomatic or respond to antacids or proton pump inhibitors. Pain from bile reflux wont' respond to such therapies, and may involve bilious vomiting. Stress-Related Mucosal Disease o In patients with severe trauma, extensive burns, intracranial disease, major surgery, serious medical diseases, and other forms of severe stress o >75% of critically ill patients develop gastric lesions during first 3 days of illness Stress ulcers: most common with shock, sepsis, or severe trauma Curling ulcers: in proximal duodenum, associated w/ severe turns or trauma Cushing ulcers: gastric, duodenal, and esophageal ulcers in patients w/intracranial disease o Pathogenesis: often related to local ischemia, or direct stimulation of vagal nuclei causing hypersecretion of acid o Morphology: range from shallow to deep erosions. Acute ulcers are round and <1cm with normal surrounding mucosa. Unlike peptic ulcers, can be found anywhere in the stomach, are often more multiple, and are not associated w/ scarring and blood vessel thickening. Healing occurs within days to weeks upon removal of injurious factors. o Clinical features: Most critically ill patients admitted to intensive care have histological evidence of gastric mucosal damage. Bleeding that requires transfusion occurs in 1-4% of patients. Chronic Gastritis o Helicobacter pylori Gastritis (most common cause) Acute infection doesn't produce symptoms in most cases, but found in 90% of chronic cases Epidemiology: associated w/ poverty and crowding, fecal-oral transmission Pathogenesis: presents predominantly antral gastritis w/ normal or increased acid production. When inflammation remains limited to the antrum, increased acid production results in greater risk of duodenal peptic ulcers. Can also progress to the body and fundus, assocaited with patchy mucosal atrophy ("multifocal atrophic gastritis"). Gastritis is the result of interplay between gastroduodenal mucosal defense, inflammatory responses, and bacterial virulence factors. Morphology: biopsy demonstrates H. pylori infection concentrated patchily within the superficial mucus, predominantly in the antrum. Mucosa is erythematous and has a coarse of even nodular appearance w/ variable number of neutrophils within the lamina propria. When intense, may create thickened rugal folds. Lymphoid aggregates are frequently present. Long-standing infection may extend to involve the body and fundus. Clinical features: tested via antibodies for H. pylori, fecal bacterial detection and urea breath test based on generation of ammonia by the bacteria urease. Can lead to peptic ulcer, adenocarcinoma, MALToma. Rx: antibiotics and proton pump inhibitors. o Autoimmune Gastritis (most common cause in patients w/o H. pylori infection) Typically spares the antrum, and is associated with hypergastrinemia Characterized by: (1) antibodies to parietal cells and intrinsic factor can be detected in serum and gastric secretions, (2) reduced serum pepsinogen I, (3) endocrine cell hyperplasia, (4) B12 deficiency, and (5) defective gastric acid secretion (achlorhydria) Pathogenesis: loss of parietal cells (loss of acid and intrinsic factor) > stimulates gastrin release > hypergastrinemia > hyperplasia of antral gastrin-producing G cells. CD4+ T cells directed against parietal cell components (H, K-ATPase aka proton pump) are considered to be the principal agents of injury. Morphology: diffuse mucosal damage of the acid producing mucosa in the body and fundus. Inflammatory infiltrate composed of lymphocytes, macrophages, and plasma cells. Clinical features: antibodies to parietal cells and to intrinsic factor are present early on. Progression to gastric atrophy over 2-3 decades. B12 deficiency can lead to anemia, seen only in a few patients (but can also lead to irreversible neurologic changes). o Uncommon Forms of Gastritis Eosinophilic Gastritis: Tissue damage associated w/ infiltration of eosinophils in mucosa and muscularis (antral/pyloric) Caused by allergic reactions (milk and soy protein most common), or via immune disorders Lymphocytic Gastritis (aka varioliform gastritis): Idiopathic, but 40% associated with celiac disease (immune-mediated) Affects the entire stomach Increased number of intraepithelial T lymphocytes Granulomatous Gastritis: Any gastritis that contains well-formed granulomas or aggregates of epithelial macrophages Gastric involvement of Crohn disaese is most common Narrowing and rigidity of the antrum may occur secondary to inflammation Complications of Chronic Gastritis o Peptic Ulcer Disease (PUD) Chronic mucosal ulceration affecting the duodenum and stomach (can be in esophagus via GERD) Nearly all are associated with H. pylori infection, NSAIDs, or smoking Most common occurs in antrum or duodenum as a result of chronic H. pylori induced gastritis (associated w/ increased acid secretion and decreased duodenal bicarbonate secretion) Epidemiology: rates dropping w/ reduced rates of H. pylori infection, but older population using increased NSAIDs has emerged Pathology: imbalance between mucosal defense and damaging factors that cause chronic gastritis Morphology: peptic ulcers most common in proximal duodenum, within a few cm of pyloric valve. Gastric peptic ulcers mostly along lesser curvature between body and antrum. Classic ulcer is a round/oval, sharply punched-out defect. Mucosal margin is usually in line with the margin of the ulcer (vs. cancer, which has elevated mucosa around its margin). Perforation into the peritoneal cavity is a surgical emergency. Bleeding from vessel walls within the ulcer base may cause life-threatening hemorrhage. Malignant transformations occur rarely. Clinical features: epigastric burning or aching pain. Some have iron deficiency anemia, hemorrhage, or perforation. Pain tends to occur 1-3 hours after meals, is worse at night, and is relieved by alkali or food. Nausea, vomiting, bloating, belching, and significant weight loss are additional manifestations. Rx: antibiotics and proton pump inhibitors, along with cessation of NSAID use. o Mucosal Atrophy and Intestinal Metaplasia Due to chronic gastritis in the body and fundus > loss of parietal cell mass Oxyntic atrophy may be associated w/ intestinal metaplasia, recognized by presence of goblet cells Risk of adenocarcinoma greatest in autoimmune gastritis o Dysplasia Chronic gastritis exposes epitehlium to free radical damage and proliferative stimuli Can lead to genetic alterations that result in carcinoma Morphology: variation in epithelial size, shape, and orientation o Gastritis Cystica Reactive epithelial proliferation associated w/ entrapment of epithelial-lined cysts Trauma-induced, can mimic invasive adenocarcinoma Hypertrophic Gastropathies o Uncommon, characterized by giant "cerebriform" enlargement of rugal folds w/o inflammation o Menetrier Disease Excessive secretion of TGFα Diffuse hyperplasia of foveolar epithelium of the body and fundus and hypoproteinemia due to protienlosing enteropathy Secondary symptoms: weight loss, diarrhea, peripheral edema Morphology: irregular enlargement of gastric rugae in body and fundus and hyperplasia of foveolar mucous cells. Inflammation is usually modest. Diffuse/patchy glandular atrophy is typical. Rx: intraveous albumin and parenteral nutritional supplementation, block TGFα growth factor receptor. o Zollinger-Ellison Syndrome Caused by gastrin-secreting tumors most commonly found in small intestines and pancreas Present w/ duodenal ulcers and chronic diarrhea, enlargement of oxyntic mucosal thickness Rx: block acid hypersecretion (proton pump inhibitors) Grow slowly, but 60-90% of gastrinomas are malignant and sporadic in 75% of patients Gastric Polyps and Tumors o Inflammatory and Hyperplastic Polyps Up to 75% of gastric polyps are inflammatory or hyperplastic polyps Chronic inflammation drives development of these polyps, highly dependent on H. pylori infection Develop in association w/ chronic gastritis, which initiates injury and leads to reactive hyperplasia Morphology: majority are smaller than 1cm and are frequently multiple o Fundic Gland Polyps Occur sporadically and in individuals w/ familial adenomatous polyposis (FAP) Prevalence increased recently due to increased use of proton pump inhibitors > increased gastric secretion > oxyntic gland growth May be asymptomatic or cause nausea, vomiting, or epigastric pain o o o o o Morphology: occur in body or fundus, well-circumscribed lesions w/ smooth surface, single or multiple, composed of cystically dilated, irregular glands lined by flattened parietal and chief cells. Inflammation is absent/minimal. Dysplasia and cancer may occur in FAP associated polyps, but not with sporadic polyps. Gastric Adenoma 10% of all gastric polyps, frequency increasing with age and in individuals w/ FAP Similar to other dysplasia, occur on background of chronic gastritis w/ atrophy and intestinal metaplasia Risk related to size of adenoma, w/ carcinoma present in up to 30% of adenomas Morphology: solitary lesions <2cm in antrum. Mostly intestinal columnar epithelium that exhibits varying degrees of dysplasia (enlargement, elongation, pesudostratification, and hyperchromasia of cell nuclei). Pre-malignant neoplastic lesions, with higher rate of transformation than intestinal adenomas. Gastric Adenocarcinoma Most common malignancy of the stomach, comprising >90% of all gastric cancers Early symptoms resemble chronic gastritis and peptic ulcer disease (dyspepsia, dysphagia, nausea) Tumors are often discovered at advance stages (weight loss, anorexia, early satiety, anemia, hemorrhage) Epidemiology: Gastric dysplasia and adenomas are precursors lesions associated w/ gastric adenocarcinomas. More common in lower socioeconomic groups and individuals w/ multifocal mucosal atrophy and intestinal metaplasia. Large rate in occurrence linked to drop in H. pylori prevalence. Pathogenesis: linked to mutation in tumor suppressor gene CDH1. Loss of E-cadherin is a key step in the development of diffuse gastric cancer. Morphology: Most involve the antrum/lesser curvature. Gastric tumors w/ an intestinal morphology tend to form bulky tumors are composed of glandular structures, while cancers with diffuse infiltrative growth patterns are more often composed of signet-ring cells. Infiltrative tumors often evoke desmoplastic reactions that stiffens the gastric wall. Large areas of infiltration display diffuse rugal flattening and rigid/thickened wall that looks like leather bottle appearance "linitis plastica" Clinical features: intestinal-type gastric cancer predominates in high-risk areas and develops from precursor lesions (flat dysplasia and adenomas). Depth of invasion and the extent of nodal and distant metastases at the time of diagnosis remain the most powerful prognostic indicators. Lymphoma Extranodal lymphomas are most commonly found in GI tract, especially stomach Referred to as lymphomas of mucosa-associated lymphoid tissue (MALT) or MALTomas Pathogenesis: usually arise at sites of chronic inflammation. Can arise at sites of preexisting MALT (ex. Peyer patches) but more commonly arise within tissues that are normally devoid or organized lymphoid tissue. In the stomach, MALT is induced as a result of chronic gastritis. H. pylori is the most common inducer. Morphology: dense lymphocytic infiltrate in the lamina propria. Infiltrate the gastric glands focally to create diagnostic lymphoepithelial lesions. Clinical features: dyspepsia and epigastric pain. Hematemesis, melena, and weight loss can also be present. Carcinoid Tumor Arise from diffuse components of the endocrine system >> "well-differentiated neuroendocrine tumors" Morphology: intramural or submucosal masses that create small polypoid lesions. Typically arise from oxyntic mucosa. Clinical features: Symptoms determined by hormones produced. Tumors that produce gastrin may cause Zollinger-Ellison syndrome, while ileal tumors may cause carcinoid syndrome > cutaneous flushing, sweating, bronchospasm, colicky abdominal pain, diarrhea, and right-sided cardiac vascular fibrosis. Most important prognostic factor is location. (1) foregut rarely metastasize, cured by resection, (2) midgut in jejunum and ileum tend to be aggressive, (3) hindgut almost always benign Gastrointestinal Stromal Tumor GI stromal tumor (GIST) is the most common mesenchymal tumor of the abdomen, >1/2 occur in stomach Pathogenesis: 75-80% of GISTs have oncogenic, gain-of-function mutations in the receptor tyrosine kinase KIT. Mutation of KIT or PDGFRA is an early event in sporadic GISTs and is detectable in lesions as small as 3mm > insufficient for tumorigenesis. Morphology: primary gastric GISTs can be up to 30cm in diameter, usually forming solitary, wellcircumscribed, fleshy mass covered by ulcerated or intact mucosa. Clinical features: 1/2 of individuals present with anemia. Rx: surgical resection. Tumors w/ KIT/PDGFRA mutation respond to tyrosine kinase inhibitors (ex. imatinib).