ESOPHAGEAL_PATHOLOGY
advertisement

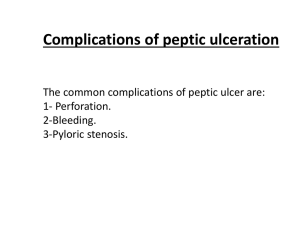
ESOPHAGEAL PATHOLOGY Esophogus: Normal Anatomy l Extends from C6 to T11/12 l 10-11 cm long in infants, 25 cm long in adults l Endoscopy measures from incisors l 3 points of contriction: u Cricoid Cartiladge u Under left mainstem bronchus u Diaphragm l No serosa; lesions easily spread Esophagus: Benign Pathology l Fistulas l Heterotopic Tissue l Rings & Webs l Diverticula l Hiatal Hernia l Lacerations l Varicies l Dysmotility CONGENITAL ANOMALIES: TRACHEOESOPHAGEAL FISTULA l Segmental absence of esophageal canalization l May end in blind pouches, or may communicate with the trachea forming a fistula. l Complications: aspiration pneumonia, asphyxia † Esophagus: Heterotopic Tissue l Gastric Mucosa u Most common just distal to the cricoid cartiladge u May ulcerate u May give rise to adenocarcinoma l Salivary Gland u RARE u Usually middle to distal 3rd ESOPHAGUS: WEBS AND RINGS l Exaggerated mucosal folds l Sx: dysphagia l Upper (above aorta): “webs” l Lower (distal 5 cm): “rings” l Plummer-Vinson syndrome: u web, glossitis, iron-deficiency anemia l Webs u Usually women u Usually > 40 yrs old l Rings u Most commonly at the EG juction u Usually 2-4 mm thick and protrude 5mm ESOPHAGUS: DIVERTICULA l Location: upper at junction with pharynx (“Zenkers”), middle & lower thirds (“Traction”) l Mechanism: acquired lumen pressure l Sx: positional regurgitation – food looks like what you ate = no digestion HIATAL HERNIA l Sliding: 90% l Rolling (paraesophageal): 10% l Symptoms: heartburn u Gastric juice reflux l Complications: bleed, infarct Esophagus: Lacerations and Varicies l Lacerations (Mallory Weiss Syndrome u Occur following fits of vomiting, most common in alcoholics u May be full thickness u Account for 5-10 % of massive hematemesis u Linear, oriented longitudinally from GE junction u Cause: relaxation failure during prolonged vomiting. u Complication: rupture left thorax (Boerhaave’s syndrome) ESOPHAGEAL VARICES (1) l Dilated valcular channels from portal hypertension (cirrhosis) l Causes massive hematemesis with a 40% fatality rate l Conditions: thrombosis of portal veins u thrombosis of hepatic veins u cirrhosis u Cancer ESOPHAGEAL VARICES (2) l Rx: u Primary prophylaxis: PharmacoRx: u l ▬ reduce portal vein pressure with beta blockers and also vasodilators. May add endoscopic ligation/thombosis. Acute bleeding: PharmacoRx: octreotide (“synthetic somatostatin”) ▬ Endoscopy: ligation or scleroRx; balloon tamponade: rescue only — “last resort” Rx (cont.): u Prevent recurrent bleeding: ▬ beta blockers plus vasodilator isosorbide mononitrate plus endoscopic procedure (ligation; scleroRx). ▬ Surgical shunts (portocaval or splenic vein anastomosis to renal veins) rapidly being replaced by pharmacoRx & endoscopy. ▬ TIPS (transjugular portosystemic shunt) now used only to “buy time” for liver transplantation (survival 85% 1 yr, 60% 5 yr). Dysmotolity: ACHALASIA l Motor dysfunction causing decreased peristalsis, incomplete relaxation of LES, and increased basal LES tone. l Other causes: Myenteric plexus absent for unknown reasons orChagas’ disease l Complications: aspiration, cancer (2-7%) l Rx: u Dilatation (but unsatisfactory) u Botulinum toxin (paralytic agent) injection into lower esophageal sphincter (experimental) u Surgical myotomy GASTRO-ESOPHAGEAL REFLUX (GERD) l Reflux of gastric content ® esophagus l Older age group l Sx: "Heartburn" u Mechanism: Esophageal sphincter tension ¯ ; delayed gastric emptying; drugs, cough l Dx: Sx, esophageal pH monitor, biopsy l Complications: Barrett's esophagus l Rx: Medical: Positional; drugs to ¯ gastric secretion. Endoscopic surgical “pleating” BARRETT ESOPHAGUS l Columnar/Gastric epithelium in esophagus l Reflux ® esophagitis ® healing ® metaplasia ® glandular epithelium l Complications: esophageal epithelium ® ulceration ® stricture ® dysplasia ® adenocarcinoma 8-10% ® no adequate, noninvasive screening test l Pulmonary complications: Acid laryngitis; asthma & pulmonary fibrosis? l “Short segment” Barrett’s = metaplasia < 3 cm from G-E junction l Complications: esophageal epithelium ® ulceration ESOPHAGUS: STENOSIS l Congenital l Acquired: u Post-inflammatory ▬ u u lye ▬ ulcer (reflux) Scleroderma (systemic sclerosis) Neoplasm ESOPHAGUS: CANCER l Cause: u mucosal erosion repair dysplasia carcinoma u Exposure: ▬ Acid reflux ▬ Alcohol (especially concentrated alcoholic drinks) ▬ Smoking = squamous cell carcinoma l Geographic frequency differences l Location: 50% middle third, 30% lower, 20% upper l Types: SCC 80-85%, adenocarcinoma 10% l Past 3 decades: incidence of adenocarcinoma (Barrett?) l Polypoid, ulcerative, diffuse l Sx: obstruction l Complications: aspiration, bleed, invasion l Invasion: tracheo-esophageal fistula; artery l Mets: nodes, late-distant l Rx: l Resectable less than 50% l ChemoRx l Radiation Rx l Prognosis: 30% 1 yr; 5-10% 5 yr Adenocarcinoma l Predominantly white and male (5:1) l Occurs most commonly in the middle to lower 3rd, and often at the GEJ. l Vast majority arise from Barrett’s. l Often appers as a nodular mass with intact epithelium. l As with SCC, usually quite advance at diagnosis. Gastric Pathology STOMACH: CONGENITAL LESIONS Diaphragmatic hernia: viscera in chest often fatal at birth (lung compression pulmonary hypoplasia) Pyloric stenosis — pyloric muscle hypertrophy Cause: ¯ nitric oxide (smooth muscle relaxant) synthase Clinical features: ○ Projectile vomiting second week ○ Visible abdominal peristalsis ○ Mass (palpation (olive), ultrasound; radiology) Male/female = 4:1; familial Rx: antral muscle incision — pyloromyotomy (Ramstedt) STOMACH: INFLAMMATORY LESIONS Acute Gastritis Causes: NSAIDS block prostaglandin synthesis microvascular injury focal mucosal necrosis ETOH smoking uremia Stress ulcers: sepsis, brain (Cushing’s) burns (Curling’s), shock, trauma Chronic Gastritis Autoimmune Gastritis Involves Fundus, Antrum usually spared Antibodies to Parietal Cells/Intrinsic Factor present ê HCL, Gastrin, and Parietal cells Significant atrophy 10% will develop pernicious anemia Associated with other autoimmune disorders Helicobacter Pylori Gastritis No associated antibodies or PA (pernicious anemia) FOUR TIMES MORE COMMON inimal fundic involvement Involves the antrum predominantly Hyperacidity, duodenal ulcers may be present. Normal Gastrin levels H. pylori has four virulence factors: Flagella (can swim in mucin) Urease (elevate local pH) Adhesins Toxins Environmental (non-specific) MOST COMMON Involves antrum and fundus Strong association with atrophy, metaplasia, and carcinoma Related to ETOH use The Histologic View: Chronic Superficial: Superficial inflammation and mild flattening Chronic Atrophic: Full thickness inflammation with atrophy of glands Gastric Atrophy No further inflammation End stage of CG PEPTIC ULCER DISEASE: CAUSES Helicobacter pylori ,NSAIDS, HCl Peptic Ulcers Usually a solitary lesion Most commonly in 1st part of duodenum (80%), lesser curvature of stomach (20%) Appear oval with over-hanging mucosal margins Nearly all have antral gastritis (HP), those who don’t are usually NSAID users 5% fatality rate (70% perf, 10% bleed) M>F Duodenal Ulcers: Genetic influences appear at work increased HCL, and gastric emptying Anterior wall of duodenum most common Gastric Ulcers: No apparent genetic influences Low to normal HCL 1-3 % delelope CA PEPTIC ULCER: DIAGNOSIS Gastric acidity (duodenal ulcer , gastric ulcer ) X-ray (5% falsely benign gastric ulcer) [Problem: benign vs. carcinoma in gastric] Endoscopy (biopsy and cytology) $ but provides definitive diagnosis PEPTIC ULCER: THERAPY Eradicate H. pylori Maintenance Rx necessary only if eradication fails or recolonization occurs If NSAIDS responsible, discontinue or substitute Antisecretory Rx Zollinger-Ellison syndrome: excise gastrinoma PEPTIC ULCER: COMPLICATIONS Bleeding (chronic anemia; massive:may die) 20%. Perforation (peritoneum; pancreas) 3-4%. Obstruction <2%. ? Cancer ? — only if in stomach and if initially ulcerating carcinoma (benign gastric ulcers do NOT become malignant over time). PEPTIC ULCER: UPPER GI HEMORRHAGE Unrelated to degree of acid production. NSAIDS = risk factor. NSAIDS and steroids = risk 10 times. Immediate Tx: Drugs don’t help. Stabilize hemodynamics Endoscopy (thermal contact, injection) Surgery (vagotomy; antrectomy) Dieulafoy’s lesion: congenital abnormally large artery in center of gastric mucosal erosion within 5 cm of gastroesophageal junction bleed Rx: Endoscopic cautery Recurrence prevention: Control H. pylori No NSAIDS HYPERTROPHIC GASTROPATHY Pathology: mucosal hyperplasia ® giant rugae Menetrier's: M/F = 4/1 mucous glands (foveolar cells)increased ; rugal folds Inc; HCl ¯ decr Hypersecretory: mucous, parietal and chief cells Incr, HCl Incr , rugal folds Incr Zollinger-Ellison syndrome: rugal folds incre, HCl and gastrin incre, increa (gastrinoma) HYPERTROPHIC GASTROPATHY ZOLLINGER-ELLISON SYNDROME Definition: gastric HCl hypersecretion and peptic ulceration due to increased serum gastrin secreted by pancreatic (sometimes in duodenal wall) islet cell tumor (gastrinoma). 50% > multiple gastrinomas 10% = multiple ulcers 60% of gastrinomas malignant (mets nodes) Age: peak 30-50 10% = MEN I (multiple endocrine neoplasia): adenomas of pancreas, pituitary, parathyroid, and adrenal cortex along with peptic ulcers Regulator gene (tumor suppressor) mutation (MEN I). Chromsome 11. Dx: serum gastrin , gastric HCL Rx: Medical: HCl control Surgery: tumor STOMACH: BENIGN TUMORS Gastrointestinal Stromal Cells Tumor (Leiomyoma) Ulcerate and bleed. Ectopic asymptomatic pancreas (mass) Polyps: Hyperplastic: benign, inflammatory, non-neoplastic Adenomatous: premalignant (carcinoma > 2 cm) Multiple polyps syndromes: ○ FAP: Familial adenomatous polyposis (neoplastic) ○ Peutz-Jeghers (hamartomas) Leiomyoma (ulcerate and bleed) Ectopic asymptomatic pancreas (mass) Polyps: Hyperplastic: benign, inflammatory, non-neoplastic Adenomatous: premalignant (carcinoma > 2 cm) Multiple polyps syndromes: ○ FAP: Familial adenomatous polyposis (neoplastic) ○ Peutz-Jeghers (hamartomas) Gastric Adenocarcinoma 85-90% of gastic ca Incidence high in asia, lower in US US incidence dropping over several decades. Risk factors: salts and nitrates with low fats Often presents at advanced stages, only 50% resectable. “Virchow’s Node,” mets to left supraclavicular node. Histologic Types Intestinal type: cells cohesive–localized, ulcerate, distal (body & pylorus), related to polyps, H. pylori, atrophic gastritis, intestinal metaplasia Diffuse type (linitis plastica): cells not cohesive; widely & deeply infiltrative, proximal (esophageal-cardial junction). 10% familial, but only 2% of all gastric Ca = E-cahedrin (CDHI) gene mutations autosomal dominant, signet-ring histology of early diffuse gastric Ca Cause: Role of H. pylori. Hereditary. Diet? Sx: 0; weight loss, pain anorexia, anemia, obstruction, Virchow’s node Dx: melena, x-ray, endoscopy with biopsy, carcinoembryonic antigen (CEA) (25%), HCl = achlorhydria in 2/3 Path: superficial (limited to mucosa an submucosa), ulcerative, polypoid, leather-bottle (linitis plastica) Tx: surgery Mets: nodes, peritoneum, liver, ovaries (Krukenberg) Prognosis: five-year survival 5-25% (but “early gastric cancer (EGC)” = 90%) Frequency: In USA, distal Ca , proximal (barrettes?) STOMACH: LYMPHOMA Frequency: 3-5% of all stomach cancer. Most common site for G.I. lymphoma H. pylori role uncertain, but probably plays a major role May be treated with antibiotics (very early if HP related) or radiation alone.