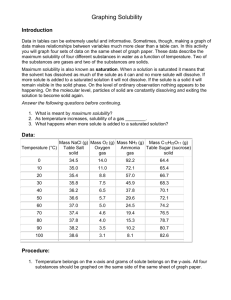
Instructions for reading solubility graphs.
advertisement

Instructions for reading solubility graphs. 1. Look at the title and legend of the graph to determine what types of solutes and solvents are used. 2. Look at x-axis to make sure the temperature is listed in Celsius . This is important if you are carrying out experiments that involve solubility. 3. Look at the y-axis to determine the solvent type and quantity used to dissolve the solute. For example, the y-axis may tell you that the graph shows how many grams of solute dissolve in 100 grams of water at a given temperature. 4. Look at the direction of the solubility curves. If a curve moves upward as the temperature increases, then the solute's solubility also increases. If a curve moves downward as temperature increases, then the solute's solubility decreases. 5. Look for the names of the solutes on the graph. These will either be written next to the solubility curves or in a legend in words or as chemical formulas. How Much Dissolves 6. Find the given temperature on the x-axis. For example, to determine how much sodium chloride -- NaCl -dissolves in 100 grams of water at 37 degrees Celsius, look on the x-axis for the 37 degree Celsius point. 7. Trace a vertical line from the point on the x-axis to the solubility curve of the solute. In this case, trace the line to the solubility curve of sodium chloride. 8. Trace a horizontal line from the intersection with the solubility curve to the y-axis. The value on the y-axis indicates the amount of solute that will dissolve in the solvent at a given temperature. For example, 38 grams of sodium chloride dissolves in 100 grams of water at 37 degrees Celsius. At What Temperature Will A Solute Dissolve 9. Find the solute and the number of grams in question. Trace a horizontal line from the point on the y axis to the solubility curve of the solute. 10. Trace a vertical line from the intersection with the solubility curve to the x-axis. The value on the x-axis indicates the temperature at which the amount of solute will dissolve in 100 mL of water. Unsaturated, Saturated, Supersaturated 11. Compare the point of intersection to the solubility curve of the solvent. If the point is below the line, then the solvent is not completely saturated at that temperature and can hold more solute; “unsaturated.” If the point of intersection is on the solubility curve then the solvent has all the solute it can handle at the given temperature: “saturated”. If the point is above the solubility curve the solvent is holding more solute than normal: “supersaturated.”