Solutions - WordPress.com
advertisement

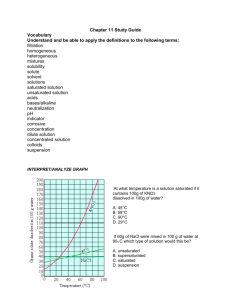
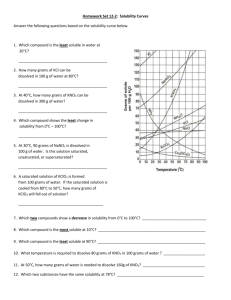
Solutions Unit 10 Pages 8-9 Learning Target: I can distinguish between unsaturated, saturated, and supersaturated solutions. I can investigate factors that influence solubilities. Criteria for Success: I can distinguish among saturated, unsaturated, and supersaturated solutions. I can define solubility. I can compare the effects of temperature and pressure on solubility. I can describe the effects of bonding and polarity on solubility. Solubility A. The __________________________ of a substance is the amount of that substance required to form a saturated solution with a specific amount of solvent at a specified _____________________________. 1. A _________________________ solution is a solution that contains the maximum amount of dissolved solute. 2. An ____________________________ solution is a solution that contains less solute than a saturated solution under the same conditions. 3. A ______________________________ solution is a solution that contains more dissolved solute than a saturated solution contains under the same conditions. Factors Affecting Solubility A. _________________________ affects the solubility of gases in liquids. 1. An _____________________ in pressure will increase the solubility of the gas. B. _________________________ affects the solubility of gases and solids in liquids. 1. An ___________________ in temperature will decrease the solubility of the gas. 2. An ___________________ in temperature will usually increase the solubility of a solid. Interpreting Solubility Graphs A. The line on the graph indicates the point at which you have a ___________________ solution of the solute indicated. 1. Any point below this line would indicate an _____________________ solution. 2. Any point above this line would still indicate a saturated solution, but one that contains ____________ solute equal to the difference between the point above the line until the point on the line at a given temperature. How Bonding Type Affects Solubility A. The type of _______________________, ________________________, and ________________________ forces will also determine solubility. “___________________________” is a rough but useful rule for predicting whether one substance will dissolve in another. 1. Liquid solutes and solvents that are not soluble in each other are __________________________. 2. Liquids that dissolve freely in one another are said to be ______________________. 4. 50 grams of which solution will settle out when it is cooled from 70◦C to 50◦C? A. NH3 B. KClO3 C. NaNO3 D. KNO3 E. KI 5. A solution of NaNO3 contains 100 gram of solute dissolved in 100 grams of water. At 50◦C this solution is considered A. unsaturated and concentrated B. unsaturated and dilute C. saturated and concentrated D. saturated and dilute Consider the solubility graph above to answer questions 1-6 1. Which substance has a solubility of 130 g at 70◦C? A NaNO3 B. KI C. NH3 D. KNO3 E. KClO3 2. Which substance is least soluble at 60◦C? A. KNO3 B. NH3 C. KClO3 D. NaNO3 E. KI 3. Which substance is most soluble at 20◦C? A. KClO3 B. NaNO3 C. KI D. NH3 E. KNO3 6. Which procedure will not increase the solubility of KI in water? A. stirring the solute and solvent mixture B. increasing the surface area of the solute C. raising the temperature of the solvent D. increasing the pressure on the surface of the solvent 7. CaBr2 will most likely dissolve in which solvent? A. CCl4 B. H2O C. C8H18 D. BI3 E. Br2 8. Under which conditions are gases most soluble in water? A. high pressure and high temperature B. high pressure and low temperature C. low pressure and high temperature D. low pressure and low temperature 9. A change in pressure would have the greatest effect on the solubility of a A. solid in a liquid B. gas in a liquid C. liquid in a liquid D. liquid in a solid 10. Which of the following solutes is likely to be most soluble in water? A. CS2 B. CH3CH2OH C. Br2 D. CCl4