Honors Chemistry WS 2.2 Key Each line in the emission spectrum of
advertisement

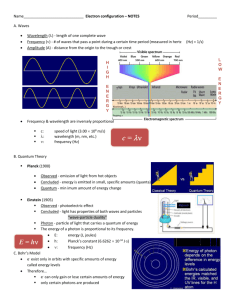
Honors Chemistry WS 2.2 Key 1. Each line in the emission spectrum of hydrogen is produced by a specific photon of light that is being emitted from the atom. When the atom absorbs electromagnetic radiation (energy) it causes an electron to become excited and it transitions (jumps) to a higher energy level. The electron falls and emits a specific photon of light that corresponds to the distance the electron falls. Since energy levels are quantized the emitted photons always have the same energy, frequency and wavelength again corresponding to the distance they have fallen. 2. The spectrum formed from white light viewed through a spectroscope contains all colors, or frequencies, and is known as a continuous spectrum. An emission spectrum shows only those colors corresponding to the absorbed and emitted photons of single gases (atoms). 3. Quantized means that electron energies can only have certain values that correspond to the distance of their transitions. It is evidence that the energy levels in Bohr’s model are at definite distances. 4. They are not continuous because the emission spectrum shows that the electron energies, colors, wavelengths, etc. are always the same for a given atom; therefore, they have to be discrete lines and discrete distances apart. 5. Excited state is when an atom absorbs energy and the electron transitions to a higher energy level. Ground state is an atom that hasn’t absorbed any energy and the electron is in its lowest energy level possible. 6. Orbital is a 3D region of space that has a high probability of containing an electron. The energy level # tells the number of orbitals. 1st – s; 2nd –s,p; 3rd –s, p, d; 4th – s, p, d, f etc. 7. 8. 1s orbital is closer to the nucleus, 2s is an orbital on the 2nd energy level, farther from the nucleus. Both, though are spherical regions of space. 9. increases 10. Energy Level (n) Number of sublevels Sublevel letter(s) # of e- in each sublevel # of orbitals in each sublevel # of e- in each orbital 1st 2nd 3rd 4th 1 2 3 4 s s,p s,p,d s,p,d,f 2 2,6 2,6,10 2,6,10,14 1 1,3 1,3,5 1,3,5,7 2 2 2 2 11. Ba: [Xe]6s2 P:[Ne]3s23p3 I:[Kr]5s24d105p5 Zr:[Kr]5s24d2 Bi:[Xe]6s24f145d106p3 12. Te 13. This is the next model after Bohr’s model was developed. The quantum mechanical model is based on quantum theory, which says matter also has properties associated with waves. According to quantum theory, it’s impossible to know the exact position and momentum of an electron at the same time. This is known as the Uncertainty Principle. The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. So, this model is based on probability rather than certainty. 14. asldf a. Pd b. Se c. Ir d. Be e. Pa 15. Electrons occupy lowest energy levels and orbitals first before filling any energy levels higher in energy. 16. Only two electrons per orbital and they must have opposite spins. 17. Every orbital in a sublevel is singly occupied with one electron before any one orbital is doubly occupied.