SPECTRAL LINES OF THE ELEMENTS
advertisement

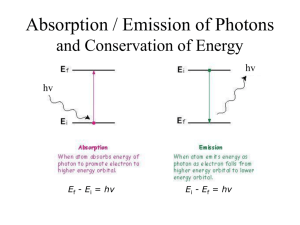
Name ______________ Pd _____ SPECTRAL LINES OF THE ELEMENTS Earth Science Lab INTRODUCTION: The three main parts of a spectrometer are the diffraction grating, the wavelength scale, and the light slit. The narrow slit allows an adequate sample of light to enter the spectrometer, and after the light passes through the slit, it strikes the diffraction grating in the eyepiece of the spectrometer. The diffraction grating is constructed of a plastic material that has hundreds of grooves etched into its surface. The diffraction grating acts like thousands of little prisms that bend the light, which causes the light to separate into the different colors of the visible spectrum. This is similar to the process that occurs when sunlight strikes rain and creates a rainbow. The wavelength scale, which is to the right of the slit, measures the length of the light waves. Due to the very small nature of the wavelength of light, we will measure with the unit of nanometers (1 nm = .000000010m or 1 x 10-9 m). The scale that you should see in the spectrometer is graduated from 350 nm to 700 nm. Carefully use the spectroscope to look at the lights in the classroom. Color the spectrum that you see, and any parts that are brighter than the rest, in the blank scale below. Note: Be precise in your illustration and line the colors up with their corresponding frequencies! CONCEPT: “Emission lines” are created when an atom’s electron loses some of the energy that it has. Remember that energy is always conserved, so if an electron loses energy, the energy that it lost MUST show up somewhere else. This energy normally leaves an atom as electromagnetic waves, and sometimes the waves that are released are part of the visible spectrum—light that we can see!!! When electrons absorb energy, they move to higher energy levels. This is called the excited state. The excited state of an atom is unstable, (the atom wants to readily change). In a fraction of a second, the electron releases the energy it has absorbed and “falls”, or moves back to its stable ground state. The energy released by the electron is in the form of photons of light. Because an atom has many different energy levels, the electrons may take many different “paths” in their return to the stable ground state—the energy released by the atom is different for each of the different paths that the electrons “follow” while letting go of the energy that they had gained. The path of the electron may be across many different energy levels at once, or it may fall from one energy level to the next lower energy level. The more energy the electron loses, the larger the release of light energy by that electron. The opposite is also true; the shorter the fall, the smaller the release of energy. Keep in mind that the amount of energy carried by electromagnetic light is revealed in the color of the light. Light at the blue/purple end of the light spectrum carries more energy (has higher frequency and shorter wavelength) than light from the red/orange end of the spectrum (which has lower frequency and a longer wavelength). Ultraviolet light carries MORE energy than blue/violet light. Infrared light carries LESS energy than red/orange light. In today’s activity, color the emission lines on the spectrometer scales for the spectral gas tubes that are displayed at the front of the room. Make sure to MATCH the colors with the correct frequency numbers! WARNING: Do NOT touch the glass tubes or the power source! NEON: What color does it glow? _______________________ HYDROGEN: What color does it glow? _______________________ HELIUM: What color does it glow? _______________________ ANSWER THE FOLLOWING QUESTIONS: Examine the three emission line diagrams that you made. Are there any exact color matches between the three gases? Does this surprise you? Explain. Does the emission spectrum that emerges in the spectroscope closely match the color that the gas glows? Why do you think this is so? Scientists examine the light released by distant stars to determine the types of elements and compounds that exist in the star. How are spectral emission lines used as a tool to identify substances?