2.1, 2.2 and 2.3 notes
advertisement
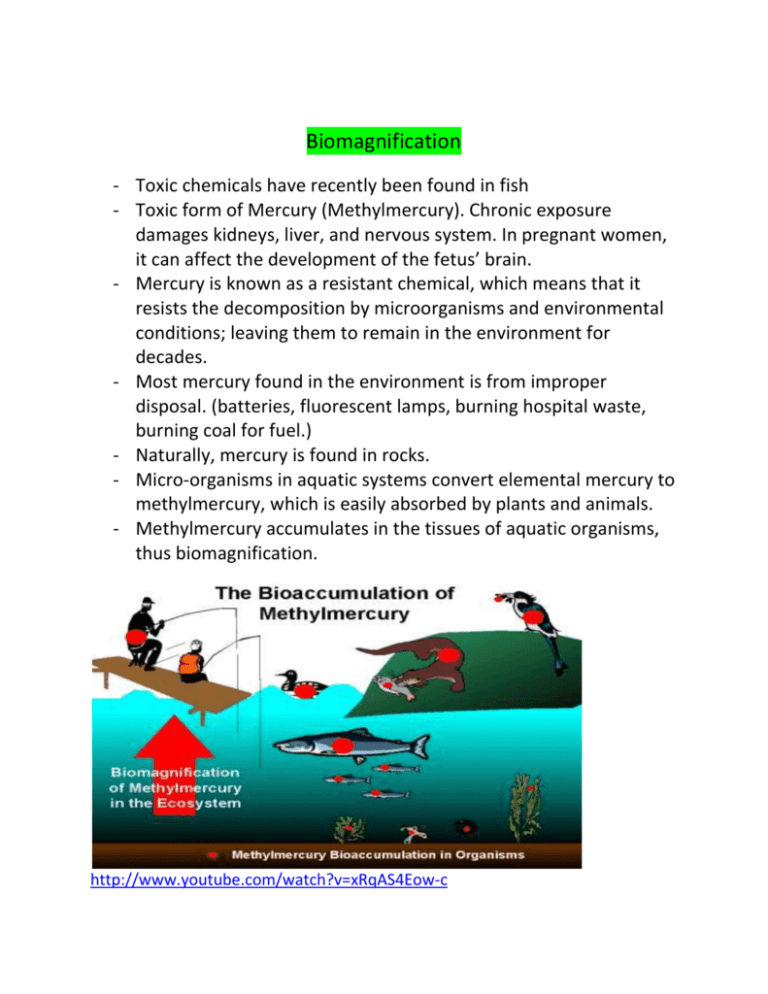
Biomagnification - Toxic chemicals have recently been found in fish - Toxic form of Mercury (Methylmercury). Chronic exposure damages kidneys, liver, and nervous system. In pregnant women, it can affect the development of the fetus’ brain. - Mercury is known as a resistant chemical, which means that it resists the decomposition by microorganisms and environmental conditions; leaving them to remain in the environment for decades. - Most mercury found in the environment is from improper disposal. (batteries, fluorescent lamps, burning hospital waste, burning coal for fuel.) - Naturally, mercury is found in rocks. - Micro-organisms in aquatic systems convert elemental mercury to methylmercury, which is easily absorbed by plants and animals. - Methylmercury accumulates in the tissues of aquatic organisms, thus biomagnification. http://www.youtube.com/watch?v=xRqAS4Eow-c Cycles of matter - Organic matter: contains hydrogen and carbon. (proteins, sugars, fats) o Broken down and rebuilt to continue cycle of matter - Matter has to be recycled o Food is organic matter Digestion breaks down complex organic molecules into simpler molecules Cells use simple molecules to build more complex molecules your body structure needs Organic molecules are held in living things and are released into the environment after death by decomposers. They break down organic molecules into inorganic molecules that pass through soil and water to be reused at a later time Water cycle (Hydrological cycle) Figure 2.2: page 35 (know this diagram) http://www.youtube.com/watch?v=0_c0ZzZfC8c&feature=related - Water is a polar molecule (The oxygen end has a slight negative charge, and the hydrogen end has a slight positive charge) - Because of this bonding of the hydrogen and oxygen, water molecules interact through hydrogen bonding o One hydrogen bonds to a nearby oxygen molecule - Water is able to remain a liquid over a large temperature range and it is able to dissolve and transport substances because of the hydrogen bonds. - Hydrogen bonds are weak individually. However, within water molecules, they form, break, and re-form frequently. - Hydrogen bonds require energy to break the bonds. Only when the bonds are broken, can water undergo a phase change from a liquid to a gas. - Cohesion: the attraction of water molecules to each other o Responsible for surface tension (why insects can walk on water) o Also keeps organic debris on the water surface http://www.youtube.com/watch?v=NVtPAaPRB3w&feature=list_related&playnex t=1&list=AVGxdCwVVULXcN_oMbBuiFyh-hT_3DkTOQ - Adhesion: the attraction of water molecules to molecules of other substances o Provides an upward force on water and counteracts the pull of gravity o Using cohesion and adhesion, water can travel from the roots of a tree up to its leaves against the force of gravity. - Water also has a high heat capacity because of its hydrogen bonding o Allowing water to heat up more slowly, and hold that temperature for longer periods Seasonal Variations in Canadian Lakes In Canada, the changing of the seasons causes significant changes in the abiotic factors in freshwater ecosystems found in our lakes. As water cools, it becomes denser, just like other substances. However, as water cools below 4°C a strange thing happens – it starts becoming less dense. When water freezes, it expands because hydrogen bonds hold the water molecules in an open crystal structure. This is why ice floats, forming a layer on top of cold water, and why the lowest layer of water in a lake often has a temperature of 4°C. When ice melts at 0°C, its solid, crystalline structure begins to break down. The loosened molecules pack more closely together and fill in the space in the collapsing solid structure. This increases the density of water, until water reaches its greatest density at 4°C. Again, this is why water at the bottom of a lake has a temperature of 4°C. Water’s density characteristics have key consequences for life and the cycling of nutrients. In the spring, as water is melting, it becomes denser as it warms until it reaches 4°C. The denser water sinks below the less dense and cooler water, leaving the cooler water at the top to warm and subsequently sink. Similarly, as winter approaches and the water temperature cools towards 4°C, the cooler water becomes denser and sinks below the warmer water. When the warmer water cools, it sinks until it reaches its maximum density at 4°C. As water sinks and rises, nutrients and dissolved oxygen are cycled with it. Assignment: Read page 35-40 Question 1 – 5 on pages 35 -40 Section 2.2 – Biogeochemical Cycles (Class Presentations) Section 2.1 Review Questions #’s 2 - 9 on p. 40 Section 2.3 The overgrowth of algae, called an algal bloom, produces large amounts of organic matter. As decomposers break down the organic matter, they use up the oxygen in the water, resulting in the death of fish and other aquatic life. Factors that contribute to algal blooms: Nutrients in the soil exposed by deforestation can be washed into rivers by rain. Sewage that is discharged into bodies of water can contain large amounts of phosphate and nitrates which promote the growth of algae. Surface run-off and snow melt carrying manure from livestock operations can add phosphate and nitrate to bodies of water. Run-off from fertilized agricultural field and enter bodies of water. http://www.dailymotion.com/video/x21ifq_algalblooms_shortfilms Read as a class pages 53 to 61 Complete p. 61 #’1-8 Assignment Phytoremediation Read p.62 Complete 1-6 (Hand in)









