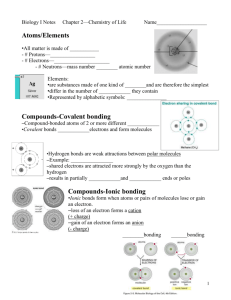
Topic 1 Water and Carbon, and Basic Components of Life
advertisement
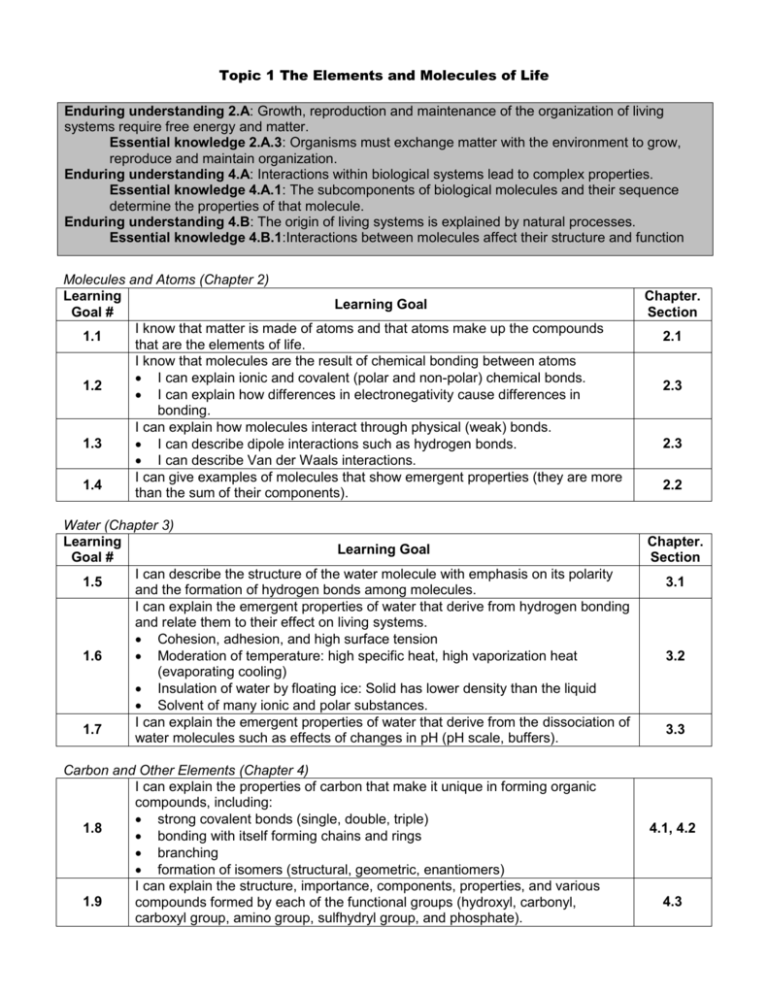
Topic 1 The Elements and Molecules of Life Enduring understanding 2.A: Growth, reproduction and maintenance of the organization of living systems require free energy and matter. Essential knowledge 2.A.3: Organisms must exchange matter with the environment to grow, reproduce and maintain organization. Enduring understanding 4.A: Interactions within biological systems lead to complex properties. Essential knowledge 4.A.1: The subcomponents of biological molecules and their sequence determine the properties of that molecule. Enduring understanding 4.B: The origin of living systems is explained by natural processes. Essential knowledge 4.B.1:Interactions between molecules affect their structure and function Molecules and Atoms (Chapter 2) Learning Learning Goal Goal # I know that matter is made of atoms and that atoms make up the compounds 1.1 that are the elements of life. I know that molecules are the result of chemical bonding between atoms I can explain ionic and covalent (polar and non-polar) chemical bonds. 1.2 I can explain how differences in electronegativity cause differences in bonding. I can explain how molecules interact through physical (weak) bonds. 1.3 I can describe dipole interactions such as hydrogen bonds. I can describe Van der Waals interactions. I can give examples of molecules that show emergent properties (they are more 1.4 than the sum of their components). Water (Chapter 3) Learning Learning Goal Goal # I can describe the structure of the water molecule with emphasis on its polarity 1.5 and the formation of hydrogen bonds among molecules. I can explain the emergent properties of water that derive from hydrogen bonding and relate them to their effect on living systems. Cohesion, adhesion, and high surface tension 1.6 Moderation of temperature: high specific heat, high vaporization heat (evaporating cooling) Insulation of water by floating ice: Solid has lower density than the liquid Solvent of many ionic and polar substances. I can explain the emergent properties of water that derive from the dissociation of 1.7 water molecules such as effects of changes in pH (pH scale, buffers). Carbon and Other Elements (Chapter 4) I can explain the properties of carbon that make it unique in forming organic compounds, including: strong covalent bonds (single, double, triple) 1.8 bonding with itself forming chains and rings branching formation of isomers (structural, geometric, enantiomers) I can explain the structure, importance, components, properties, and various 1.9 compounds formed by each of the functional groups (hydroxyl, carbonyl, carboxyl group, amino group, sulfhydryl group, and phosphate). Chapter. Section 2.1 2.3 2.3 2.2 Chapter. Section 3.1 3.2 3.3 4.1, 4.2 4.3