Unit 1-2 Review Key
advertisement

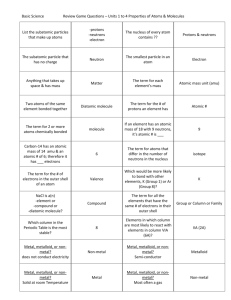
Practice Problems: KEY 1. Ms. Jensen wants to know which location in her house is best for growing basil plants. She has three basil plants. She puts one on the balcony, one by the kitchen window, and in a closet. Each plant has the same size pot and the same soil, and she gives the plants the same amount of water. a. Which is the independent variable? c. What controls has she built into the experiment? Location of the plant b. Which is the dependent variable? Soil, pot size, amount of water Growth of the plant 2. At the beginning of a star’s life, which two elements is it made of? Hydrogen and Helium 3. How are elements heavier than hydrogen created in stars? Fusion 4. What is the last element that can be created in a star by the normal fusion process? Iron 5. How are the rest of the elements of the periodic table formed (after the normal fusion process)? From the energy of a SUPERNOVA! 6. Identify each of the following changes as either a physical change (PC) or a chemical change (CC). a. PC A metal rod is cut in half e. CC Bubbles of H2 gas rise to the b. CC Bread gets moldy surface when Li is put in water c. PC A cup of water evaporates f. PC A pencil is sharpened d. CC A match is lit 7. Classify each of the following properties of matter as either a physical property (PP) or a chemical property (CP): a. CP Burns easily (flammable) d. PP Ductile b. PP Density e. PP Brittle c. PP Boils at 200 oC f. CP Reacts with oxygen 8. Classify each of the following substances as a mixture (M), compound (C), or element (E). a. E Silver (Ag) e. C Brass (= Cu + Zn) b. M Kool-aid f. M Soil, or dirt c. C Carbon Dioxide (CO2) g. C Methane (CH4) d. M Paper h. E Gallium (Ga 9. An unknown element is brittle, breaking when hit with a hammer. Is it a metal, metalloid, or nonmetal? 10. An element is a good conductor of electricity. Is it a metal, metalloid, or nonmetal? 11. An element is malleable and ductile. Is it a metal, metalloid, or nonmetal? 12. An element is a poor conductor of heat. Is it a metal, metalloid, or nonmetal? 13. Find the density of a material, given that a 2.00-g sample occupies 9.50 mL. d = m/v = 2.00 g / 9.50 mL = 0.21 g/mL 14. Use the periodic table to write the information for the following elements. Identify each as a metal, nonmetal, or metalloid. Symbol Name Na Sodium Chlorine Boron Krypton Silver Beryllium Lead Sulfur Cl B Kr Ag Be Pb S Group Number 1 17 13 18 11 2 6 3 Period Number 3 3 2 4 5 2 6 3 Metal, Nonmetal, or Metalloid Metal Nonmetal Metalloid Nonmetal Metal Metal Metal Nonmetal Family (or other section) Name Alkali Metals Halogen Metalloid Noble gas Transition Metals Alkaline Earth Metals Other Metals / None Non-metals 15. A sample of a substance that has a density of 6.25 g/mL has a mass of 50.1 g. Calculate the volume. Use algebra to get variable by itself: v•d = m/v•v dv/d = m/d v = m/d v = m/d = 50.1 g / 6.25 g/mL = 8.016 mL 16. A student measures the mass of a sample as 10.0 g. Calculate the percent error if the correct mass is 9.75 g. | 9.75 g – 10.0 g | x 100 = 2.56% 9.75 g 17. A handbook gives the density of water as 1.0 g/cm3. What is the percent error of a density calculation of 0.90 g/cm3 based on lab measurements? | 1.0 g/mL – 0.90 g/mL | x 100 = 10% 1.0 g/mL 18. Write the following in decimal notation or scientific notation a.) 6.70 × 10-3 0.0067 a.) 235 000 2.35 x 105 b.) 9.112 × 105 911,200 b.) 0.000 002 320 2.320 x 10-7 c.) 1.4 × 103 1,400 c.) 56 000 000 5.6 x 107 19. Convert 200 meters (m) to of inches (in). Conversion Factors: 2.54 cm = 1 inch, 1 m = 100 cm 200 m | 100 cm | 1 inch = 7874.02 inches 1m 2.54 cm 20. Convert 465.1 meters per second (m/s) to miles per hour (mi/hr). (Interesting fact: This is how fast the Earth spins!) There are other ways to calculate this, such as converting meters to cm to inches to feet to miles. This is just one way of doing it. If you do it correctly on the test and still arrive at the correct answer you will earn full points. Conversion Factors: 3600 sec = 1 hour, 1000 m = 1km, 1.61 km = 1 mile 465.1 m | 100 cm | 1 inch = 7874.02 inches sec 1m 2.54 cm 19. Convert 12.43 weeks to units of seconds. (This is how far away Christmas is.) Conversion Factors: 60 sec = 1 hour, 60 sec = 1 min, 7 days = 1 week, 1 day = 24 hours 12.43 weeks | 7 days | 24 hours | 60 minutes | 60 seconds = 7.517664 x 106 seconds 1 week 1 day 1hour 1 min