work sheet 2
advertisement

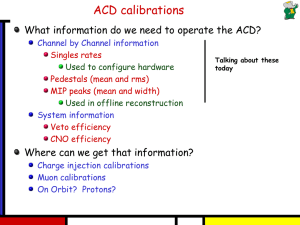
Kingdom of Saudi Arabia Ministry of Higher Education Preparatory Year Deanship Work sheet (2) Determine each of the following is metal , nonmetal or metalloid: Properties Metal Nonmetal Metalloid Insulators Good conductor for electricity Semiconductors Brittle Ductile Malleable Determine number of protons, electrons and neutrons: Atom 45 21 Sc 55 25 Mn 52 24 Cr protons electrons neutrons 𝟔𝟓 𝟐𝟗𝒄𝒖 Give an one example from the following set ( Cl , Ag+ ) for the following: metal nonmetal cation 107 109 47 47 anion 1 Ag , Cu2+ , Na , F - , OH¯, isotopes Polyatomic ions Ag Calculate the average atomic mass for the Rubidium which has two isotopes: Isotope 85 Rb 87 Rb Abundance 72.17 % 23.83 % Mass 84.9118 86.9092 ……………………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… ……………………………………………………………………………………… ………………………………………………………………………………………. What the formula of compound formed between 1) Iron (III) Fe 3+ and sulfide (S2-) ……………………………………………………………………………… ……………………………………………………………………………… 2) Ammonium (NH4)+ and phosphate (po4)3..................................................................................................................................... .................................................................................................................................... 3) Tin ( Sn+2 ) and oxide ( O-2 ) ……………………………………………………………………………… ……………………………………………………………………………… 4) Manganese (Mn+4 ) and oxide (O-2 ) ……………………………………………………………………………… ……………………………………………………………………………… 2 5) Cu+1 and O-2 ……………………………………………………………………………… ……………………………………………………………………………… 6) Cu+2 and O-2 ……………………………………………………………………………… ……………………………………………………………………………… 7) 𝑁𝐻4+ 𝑎𝑛𝑑 𝑆𝑂3−2 ……………………………………………………………………………… ………………………………………………………………………………. 3 write the name of each the following compound: 1) 2) 𝐹𝑒2 𝑂3 ……………………………………………………………………………… ……………………………………………………………………………… Cu SO4 . 6 H2O ……………………………………………………………………………… ……………………………………………………………………………… 3) KCl : ……………………………………………………………………………… ……................................................................................................................ 4) NF3 : ……………………………………………………………………………… ……………………………………………………………………………… 5) MgSO4 . 7H2O : ……………………………………………………………………………… ……………………………………………………………………………… 6) CaO : ……………………………………………………………………………… ……............................................................................................................... 7) CS2 : ……………………………………………………………………………… ……………………………………………………………………………… 8) Na2CO3∙10H2O : ……………………………………………………………………………… ………………………………………………………………………………. 9) NaBr : ……………………………………………………………………………… ……................................................................................................................ 10) PCl5 : ……………………………………………………………………………… ……………………………………………………………………………… Good luck 4 5