Stoichiometry powerpoint
advertisement

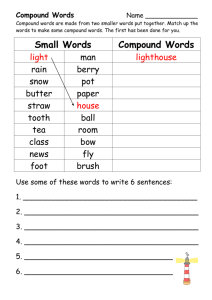
AP Chem Cumulative Review Stoichiometry Composition Stoichiometry 1. What information can be derived from a chemical formula of an ionic compound versus a molecular compound? 2. What three conversion factors and in what order would you use them to convert the mass of a compound into atoms of a particular element in that compound? Calculate the number of hydrogen atoms in a 1-g sample of aspirin (C9H8O4). 5. …illustrates the schematic diagram of a combustion device used to analyze organic compounds. Explain how the data relating to the mass of CO2 produced and the mass of H2O produced can be manipulated to determine the empirical formula. A compound contains only C,H, and O. Combustion of 10.68 mg of the compound yields 16.01 mg CO2 and 4.37 mg H2O. The molar mass of the compound is 176.1 g/mol. What are the empirical and molecular formulas of the compound? • A 0.755-g sample of hydrated copper(II) sulfate CuSO4 xH2O was heated carefully until it had changed completely to anhydrous copper(II) sulfate with a mass of 0.483g. Determine the value of x, also called number of water of hydration. It specifies the number of water molecules per formula unit of CuSO4 in the hydrated crystal. Reaction Stoichiometry 4. Give the balanced equation: 6. Consider the hypothetical reaction between A2 and AB pictured below: • What is the balanced equation? • If 2.5 mol A2 is reacted with excess AB, how many moles of product will form? • If the mass of AB is 30 amu and the mass of A2 is 40 amu, what is the mass of the product? • If 15g of AB is reacted, what mass of A2 is required to react with all of the AB, and what mass of product is formed? 7. Consider the following mixture of SO2 and O2: • If SO2 and O2 react to form SO3, draw a representation of the product mixture assuming the reaction goes to completion. What is the limiting reactant in the reaction? • If 96g of SO2 reacts with 32g O2, what mass of product will form? 8. In an experiment, varying masses of sodium metal are reacted with a fixed initial mass of chlorine gas. The results of the experiment are shown by the graph below: Explain the general shape of the graph and estimate the amount of chlorine gas used in this experiment. 9. A sample of limestone, calcium carbonate, and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide: A 1.506g sample of limestone-containing material gave 0.558g of CO2 in addition to CaO, after being heated at a high temperature. What is the mass percent of CaCO3 in the original sample? This statue of George Washington was first put outside in New York City in 1944. During the next 58 years, acid rain caused significant damage to the statue.