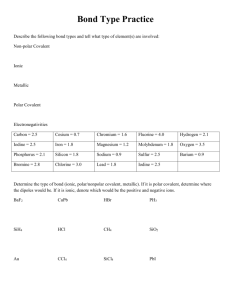
Name Ionic, Covalent, Metallic, and Polarity 1. Which bond type
advertisement

Name _________________________________ Ionic, Covalent, Metallic, and Polarity 1. Which bond type usually occurs when a non-metal bonds with another non-metal? A. covalent B. ionic C. metallic 2. How do electrons in a metallic bond behave? A. electrons flow freely between atoms B. electron number is reduced C. atoms gain or lose electrons D. atoms share pairs of electrons 3. How do electrons in an ionic bond behave? a. electrons flow freely between atoms b. atoms gain or lose electrons c. atoms share pairs of electrons 4. How do electrons in a covalently bonded substance behave? A. electrons flow freely between atoms C. atoms gain or lose electrons B. each electron is associated with only one atom D. atoms share pairs of electrons 5. A solid does not conduct electricity. When it dissolves in water, the solution does not conduct electricity? What type of compound is it? A. covalent B. ionic C. metallic 6. A student tests a white, crystalline solid and finds that it does not conduct electricity as a solid. It does conduct electricity when dissolved in water, and has a very high melting point. The substance is most likely A. a covalent compound C. a metallic compound B. an ionic compound D. a semi-metallic compound 7. Which material would be most malleable? A. At B. Se C. Si D. Pd A student tests several different solid compounds for solubility and conductivity. The data are shown in the chart below. Use them to answer the next three questions. Material Solubility Conductivity of Conductivity of in water Solid water when substance is placed in water Substance A Soluble none high Substance B Soluble none none Substance C Insoluble high low 8. Based on the data above, which substance most likely has ionic bonds? A. Substance A B. Substance B C. Substance C 9. Which material most likely has covalent bonds? A. Substance A B. Substance B C. Substance C 10. Which compound most likely has metallic bonds? A. Substances A B. Substances B C. Substances C Use the following diagrams to answer the next three questions. 11. What is the shape and polarity of methane (CH4)? A. tetrahedral, polar C. linear, non-polar B. tetrahedral, non-polar D. pyramidal, polar 12. What is the shape and polarity of a water molecule? A. bent, nonpolar C. Linear, nonpolar B. pyramidal, polar D. Bent, polar 13. What is the shape and polarity of a molecule of ammonia (NH3)? A. tetrahedral, non-polar C. pyramidal, non-polar B. pyramidal, polar D. Bent, polar 14. Symmetrical molecules are usually: A polar B. non-polar 15. If chlorine is more electronegative than iodine, compare the boiling point of HCl to HI A. HCl would become a gas at a higher temperature compared to HI B. HCl would become a gas at a lower temperature compared to HI C. They would both become gases at the same temperature D. Both are gases at room temperature so their boiling points can’t be compared 16. When water boils A. a chemical reaction takes place B. intermolecular bonds are broken and individual water molecules separate from each other C. covalent (intramolecular) bonds are broken D. the hydrogen atoms separate from the oxygen atoms to form H2 and O2 gas 17. If oxygen is more electronegative than nitrogen, how would the physical properties of NH3 compare to H2O? A. NH3 will evaporate more slowly than H2O if they are kept at the same temperature B. NH3 will evaporate more quickly than H2O if they are kept at the same temperature 18. Why do belly-flops hurt? a. water is a polar molecule b. water has a high heat capacity c. water is a covalent compound d. water has a low molecular weight