Chemistry & Physics Worksheet: Properties, Energy, Matter
advertisement

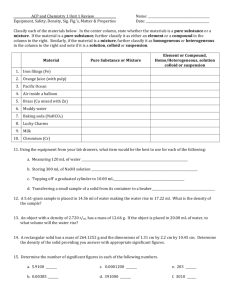
1. Classify each of the following properties as chemical or physical: color chemicalphysical stability chemicalphysical resistance to corrosion chemicalphysical Classify the underlined property as chemical or physical: 2. Plants use carbon dioxide from the air to produce oxygen. Iodine is a purple solid that melts at 114 oC. chemicalphysical chemicalphysical The white solid calcium carbonate gives off carbon dioxide gas when exposed to acid. chemicalphysical chemical Plants use carbon dioxide from the air to produce oxygen. chemical physical Iodine is a purple solid that melts at 114 oC. physical The white solid calcium carbonate gives off carbon dioxide gas when exposed to acid. chemical chemical 3 3. Classify each of the following changes as chemical or physical: grilling a steak souring of milk adding antifreeze to a car radiatior 4. State whether the kinetic energy of the underlined object increases or decreases as a result of the change described. an archer shoots an arrow increases increases a golf ball lands on a green decreases decreases a bullet is shot from a pistol increases increases 5. State whether the potential energy of the underlined object increases or decreases as a result of the change described. a positive ion and a negative ion are separated a cat climbs a tree a skier skis from the top to the bottom of a hill 3 3 6. 3 Which the following best describe the matter in the picture increases increases increases increases decreases decreases Pure substance Heterogeneous mixture Homogenous mixture 7. Classify each of the following as either a pure substance or a mixture: apple juice ethyl alcohol tooth paste 8. Classify each of the following mixtures as either homogeneous or heterogeneous: gasoline chocolate chip ice cream cider vinegar 9. Classify each of the following as either a pure substance a homogeneous mixture or a heterogeneous mixture: ibuprofen sand and salt maple syrup 10. Classify each of the following as either a pure substance a homogeneous mixture or a heterogeneous mixture: ibuprofen heterogeneous pure sand and salt heterogeneous heterogeneous maple syrup homogeneous homogeneous 11. Classify each of the following pure substances as either an element or a compound: sugar aluminum potassium nitrate 12. Classify each of the following pure substances as either an element or a compound: 3 3 NaCl compound Cu element KNO3 compound 3 3 3 3