Short Answer - Ms Brown`s Chemistry Page
advertisement

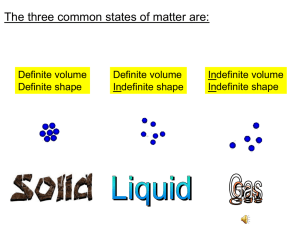
Name __________________________________________ Block ____________ Date ____________________ Chemistry 543 Review: Chapter 1-The Science of Chemistry Fill in the blank physical change chemical change elelment chemical property physical property heterogeneous mix solution weight density compound 1. A change that produces one or more new substances is known as a chemical change. 2. The simplest pure substance is a(n) element. 3. The ratio of mass to volume is defined as the density of matter. 4. A property of matter that can be measured without changing its chemical nature is known as a physical property. 5. Two or more elements that are chemically combined are known as a(n) compound. 6. Oil and water mixed for a salad dressing and left standing is an example of a heterogeneous mix. 7. Sugar dissolved in water is an example of a solution. Short Answer 8. Explain why gases and liquids take the shape of their containers using particle model descriptions. there is little to no attraction between molecules. 9. What is the difference between an endothermic reaction and an exothermic reaction? endothermic reactions absorb energy and feel cool to the touch while exothermic reaction release energy in the form of heat and/or light. 10. List the four indicators that signify that a chemical change has taken place. 1. form precitpitate 2. form gas 3. release or absorb energy (heat/light) 4. change in color 11. What is the difference between mass and weight? mass is the amount of matter in an object while weight is the force of gravity on matter. 12. Convert the following measurements, show your work. a. 250 micrograms (ug) to kilograms (kg) 𝟏 𝒌𝒈 250 ug x 𝟏,𝟎𝟎𝟎,𝟎𝟎𝟎,𝟎𝟎𝟎 𝒖𝒈 = 2.50 E -7 kg b. 4.3 liters(L) to cubic centimeters (cm3) 4.3 L x 𝟏𝟎𝟎𝟎 𝒄𝒎𝟑 𝟏𝑳 = 4300 cm3 c. 200 cm to m 𝟏𝒎 200 cm x 𝟏𝟎𝟎 𝒄𝒎 = 2 m d. 0.09 ks to ms 0.09 ks x 𝟑𝟓 𝒎𝒊 𝟏 𝒉𝒓 𝟏,𝟎𝟎𝟎,𝟎𝟎𝟎 𝒎𝒔 𝟏 𝒌𝒔 = 90,000 ms e. 35 mi/hr to m/s 𝟏 𝒉𝒓 x 𝟏 𝒎𝒊 x 𝟑𝟔𝟎𝟎 𝒔 = 15.6 m/s 𝟏𝟔𝟎𝟗 𝒎 13. What is an allotrope? Give an example. element with more than one form in the same physical state examples: carbon - diamond and graphite oxygen - ozone and molecular oxygen 14. Classify the following materials as heterogeneous mixtures, solutions, compounds or elements: a. concrete hetero mix b. sodium element c. baking soda compound d. table salt compound e. aluminum foil element f. gravel hetero mix g. brass solution h. milk solution i. apple hetero mix j. kool aid solution 15. Classify the following properties as chemical or physical: a. heat conductivity physical b. combustibility chemical c. resistance to acids chemical d. length physical e. brittleness physical f. malleability physical 16. Classify the following changes as chemical or physical: a. silver tarnishing chemical b. melting butter physical c. milk souring chemical d. wood burning chemical e. water evaporating physical 17. Be sure to know your elements…..there will be a 10 point question on the exam. Use the flash cards as well as the matching game, chemistry mahjong.