Unique Properties of Water jigsaw
advertisement
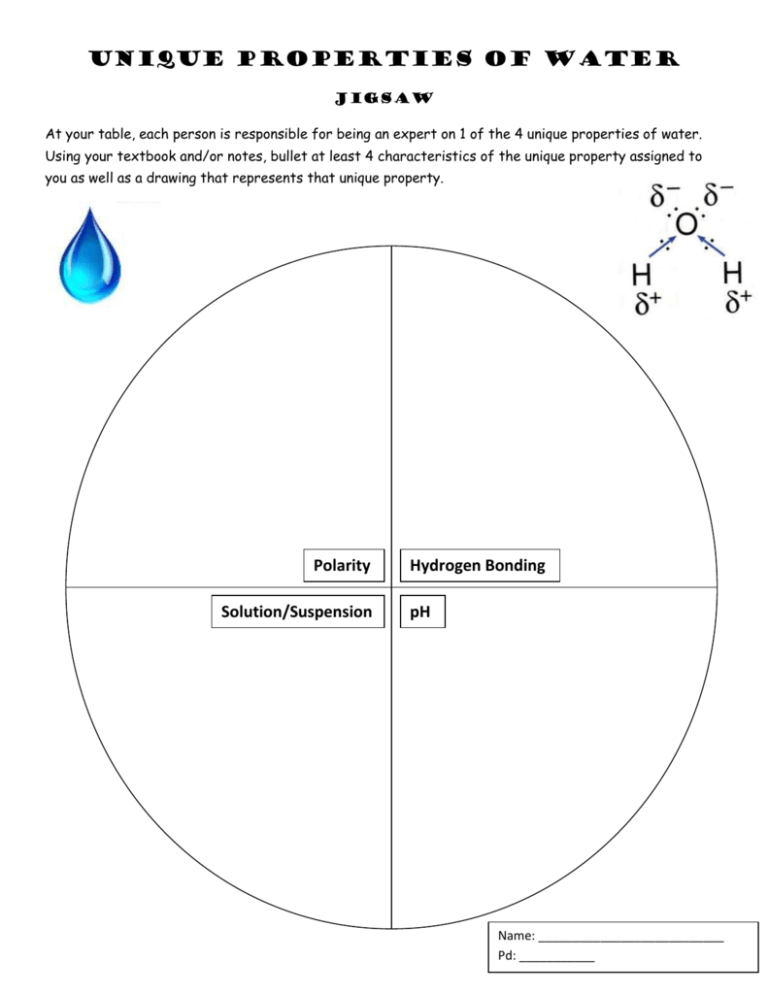
Unique Properties of Water Jigsaw At your table, each person is responsible for being an expert on 1 of the 4 unique properties of water. Using your textbook and/or notes, bullet at least 4 characteristics of the unique property assigned to you as well as a drawing that represents that unique property. Polarity Solution/Suspension Hydrogen Bonding pH Name: ___________________________ Pd: ___________ CH. 2-2 Properties of Water Homework 1) POLARITY a. What makes water a polar molecule? _______________________________________________ b. Which end of the water molecule attracts most of the electrons and acts negative? __________ c. Which end acts positive? _______ 2) a. b. c. HYDROGEN BONDING What is a hydrogen bond? _________________________________________________________ How do water molecules act like “little magnets”? ______________________________________ What is heat capacity and how is it important to the human body? _________________________ 3) a. b. c. COHESION What is cohesion? ________________________________________________________________ Cohesion produces _____________ tension as the water molecules hold tightly together. How do living things like insects use surface tension? ____________________________________ 4) a. b. c. ADHESION Define adhesion. __________________________________________________________ Adhesion produces an effect called ______________ action when you place a straw in a liquid. Explain how adhesion helps plants move materials. _________________________________________ 5) a. b. c. d. SOLUTIONS When making Kool-AID, which part is the solvent? _____________ solute? ____________________ Salt dissolved into water is an example of? Circle one solution/suspension Oil and water is an example of? Circle one solution suspension Matching: Solution _____ a. heterogenous mixture Suspension _____ b. homogenous mixture 6) UNIVERSAL SOLVENT a. Which type of molecules, polar or nonpolar, dissolves in water? ________________________ (hint: like dissolves like) b. Are materials that dissolve in water hydrophobic or hydrophilic? _________________________ c. 3 examples of compounds water easily dissolves: ____________________________ ____________________________ ____________________________ d. How does water’s polarity influence its properties as a solvent? ________________________________ _______________________________________________________________________________________ 7) DENSITY a. What happens to hydrogen bonds when water freezes? __________________________ __________________________________________________________________________ b. Why is this important to organisms living in the Arctic? __________________________ ___________________________________________________________________________ WHAT TO INCLUDE IN YOUR POSTER: Write the Poster title - PROPERTIES OF WATER In the Upper right corner, write all your group member’s names and period You are to include all of the following tasks on your poster. TASK 1: POLARITY Draw and color (Oxygen-RED and Hydrogen- BLUE) water molecule and the valence electrons of oxygen and hydrogen. Draw in where the slight negative and slight positive charges are on the molecule below. o On the back side of your jigsaw, answer the questions under POLARITY. TASK 2: HYDROGEN BONDING Cut out the image below and paste it on your poster. Color (Oxygen-RED and Hydrogen- BLUE) water molecules held together by hydrogen bonds. Add ARROWS pointing to the all the hydrogen bonds in this image. On the back side of your jigsaw, answer the questions under HYDROGEN BONDING TASK 3: COHESION Either cut out the image to the right and color it OR draw your own representation of this image on the poster. Label the image COHESION. On the back side of your jigsaw, answer the questions under COHESION. TASK 4: ADHESION Cut out and color the picture below, color the liquid BLUE. Paste it on your poster and label it ADHESION. Adhesion pulls the liquid up the sides of the straw. On the back side of your jigsaw, answer the questions under ADHESION. TASK 5: SOLUTIONS Cut out the image to the right and paste it on your poster. Label the solute and solvent. Color the solute and solvent. On the back side of your jigsaw, answer the questions under SOLUTIONS. TASK 6: UNIVERSAL SOLVENT Cut out and color the images below. Color Cl GREEN, Color Oxygens RED, color H BLUE, and color Na purple. On the back of your jigsaw, answer the questions under UNIVERSAL SOLVENT TASK 7: DENSITY Draw a picture of a glass of soda with ice in it. On the back of your jigsaw, answer the questions under DENSITY.