Atoms and Bonding
advertisement

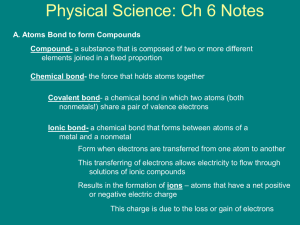
Physical Science STP Chemistry Unit 2 (Ch5-8) Study Guide Atoms and Bonding Compounds are formed by combining two or more different elements. Compounds have properties that are different from their constituent elements. Valence electrons are the electrons of an atom that are at the highest energy level and are more loosely held to the atom. It is these electrons that make up chemical bonds Ionic Bonds Ions, atoms or groups of atoms with a positive or negative charge (from losing or gaining electrons) are formed in a process called ionization. Ionic Bonds are bonds made between ions of different elements through chemical reactions. These bonds occur as a result of the attraction between positive and negative ions. A compound consisting of ionic bonds is called an ionic compound. Ionic compounds usually consist of a metal bonding to a non-metal. Ionic bonding can be visualized with the aid of Lewis diagram Properties of ionic compound are in general, hard, brittle solids with high melting points. These compounds when melted or dissolved in water conduct electric current. Covalent Bonds A chemical bond that is formed when two atoms share electrons is called a covalent bond. The force that holds these atoms together in a covalent bond is the attraction of each atom's nucleus for the shared paired of electrons. The resulting neutral group of atoms is called a molecule. The compound formed is called a molecular compound and they usually consist of a non-metal to a non-metal. Molecular compounds generally have lower melting points and boiling points, and do not conduct electric current when melted or dissolved. H2O Metallic Bonds Metal atoms combine in regular patterns in which the valence electrons are free to move from atom to atom. Chemical Reactions Evidence for chemical reactions involve changes in properties and energy that can be observed. Examples include formation of a precipitate, color change, and gas produced from a liquid or solid reactant. Chemical reactions are indicated by chemical equations. Chemical equations use chemical formulas (symbols of elements combined together to show a compound. ex NaCl) and other symbols instead of words to depict a reaction. An equation shows two sides, the one on the left being the compounds reacting together (REACTANTS) and the one on the right are the compounds that are formed (PRODUCTS) from the reaction. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(l) Chemical equations are balanced by conserving matter. The number of atoms on each side of the equation must be equal for each type of element represented. Chemical reactions are classified by three general types which are synthesis, decomposition and replacement (single or double). The classifications refer to the results of a reaction. Synthesis - a new compound is formed from two single elements. Decomposition a compound is broken down into simple elements. Replacement - when one or two elements replace another (trade places). Reactions absorbing energy are called endothermic reactions, and those releasing energy are called exothermic reactions. Rates of reactions can be affected by surface area, temperature, concentration, and the presence of catalysts or inhibitors. Solutions Solutions contain a solvent and a solute. The solvent is the part that dissolves other substances. The solute is the part that is dissolved by the solvent. The amounts of solute or solvent in a solution can vary, this is referred to as concentration. Solutes lower the freezing point and raise the boiling point of a solvent. An important property of solvents is how much a solvent can dissolve certain solutes. This is called solubility. There are three classifications of solubility. 1) Saturated - there is just enough solute in the solution so that it all dissolves. 2) Unsaturated - there is less solute in the solution than can be dissolved by the solvent. 3) Supersaturated - there is more solute in the solution than the solvent can dissolve. Factors that affect solubility include pressure, temperature and type of solvent. Acids and Bases An acid or a base are a compounds whose characteristic properties include the kinds of reactions they undergo. A numerical scale was developed to help identify acids and bases - pH scale. Acidic properties include sour taste, reacts with metals (corrosive) and carbonates and turns blue litmus paper red. On the pH scale acids range from a value of 1 (being strong) through 6 (being weak). Acids contain hydrogen ions (H+) in solution. Basic properties include bitter taste, slippery feel, turns red litmus paper blue. On the pH scale bases range from 1214 (being strong) down to 8 (being weak). A value a 7 is considered neutral. Water is an example of a neutral solution. Bases contain hydroxide ions (OH-) in solution. Carbon Carbon is the element of life, and it is the most important element in organic chemistry. Carbon is capable of forming up to 4 covalent bonds. Can create long chains: straight, branched, rings. Can bond with many elements. Has four pure forms: Graphite, Diamond, Fullerenes, and Nanotubes Organic = From living things. Inorganic = From non-living things. Remember: Life is made up of Schnop! The elements of life: Sulfur, Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus