Bonding
advertisement

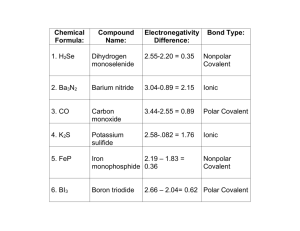
Bonding By Mary Agarwala Chemical Bonds? What are they? Why do atoms form them? Why are some stronger than others? Ionic Bonding + charged ion is attracted to a – charged ion A nonmetal + a metal = ionic bond Ionic compound is entirely made up of ions Positively charged ion (Cation) Negatively charged ion (Anion) Ex. NaCl Ionic properties High melting points which means it ionic bonds are strong bond Brittle Dissolves in water, a process that breaks the ionic bonds and separates the ions (makes solutions that conduct electricity) Polar…”Likes dissolves likes” Liquid (motlen) ionic compounds conduct electricity Solid ionic compounds do not conduct electricity well OCTET RULE Atoms tend to gain, lose, or share electrons in order to acquire a full set of valence electrons Lewis Dot Diagrams Valence electrons are represented as dots placed around the element symbol KNOW HOW TO WRITE IONIC COMPOUNDS Empirical formula vs. Molecular formula Empirical Formula Denote the ratio of ions in a compound Chemical formula Molecular Formula Molecular compound Describes the composition of a molecular compound The lowest possible whole Tells how many atoms are in The compound wants to be Ex. Glucose (C6H12O6) number subscripts for the elements electrically neutral a single molecule of the compound Covalent Bonds -A covalent bond is formed by a shared pair of electrons between two atoms -Each atom wants to be “octet happy” -Nonmetal + nonmetal = Covalent bond -This is best shown by combining atoms’ Lewis structures Multiple Bonds Double Bonds Triple Bonds (show picture) (show picture) (text book pg 239. Eq.4) (text book pg. 239 Eq.5) Exceptions to the Octet Rule Atoms with less than an octet (BF3) Atoms with more than an octet (SF4) Molecules with an odd number of electrons (NO) Don’t sweat the exceptions for the regents Electronegativity Property of an element that indicates how strongly an atom of that element attracts electrons in a chemical bond When one atom is SIGNIFICANTLY MORE EN than another, the covalent bond is POLAR. 2 atoms, in a bond, with SIMILAR ENs are NONPOLAR KNOW EN TRENDS!!! Remember F is the biggest (EN=4.0) Bond Type by Electronegativity Electronegativity Difference Less than or equal to 0.4 Between 0.4 and 2.0 Greater than or equal to 2.0 Bond Type Nonpolar covalent Polar covalent ionic Naming Chemical Compounds Chemists name a compound according to the atoms and bonds that compose it Write cation first Naming Ionic Compounds No need to specify number of atoms (AKA no prefixes) Ex. KI (potassium iodide) Ex. KNO3 (potassium nitrate) Ex. CuSO4 (copper(1) oxide) Naming Molecular Compounds Know numerical prefixes Suffix –ide is added to the atom with a greater EN Ex. NO2 (nitrogen dioxide) Ex. BF3 (boron trifluoride) Ex. P2O5 (diphosphorus pentoxide) THE END “Good Luck.. Do Well!!!!”