lec24_2013 - Andrew.cmu.edu
advertisement
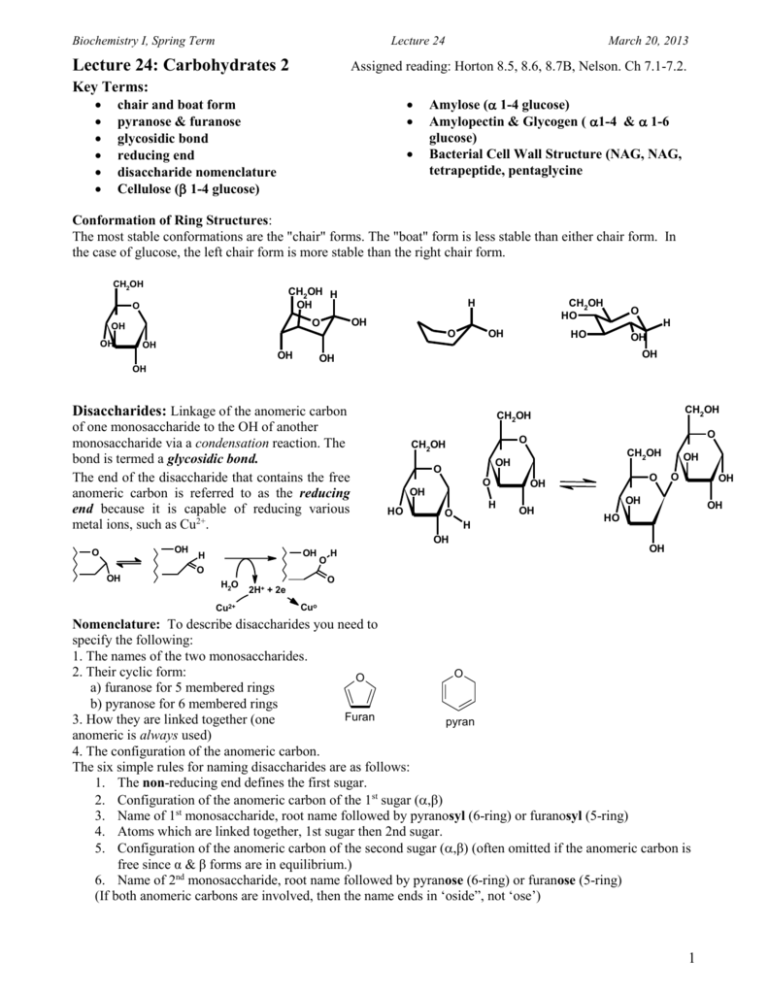
Biochemistry I, Spring Term Lecture 24 Lecture 24: Carbohydrates 2 March 20, 2013 Assigned reading: Horton 8.5, 8.6, 8.7B, Nelson. Ch 7.1-7.2. Key Terms: chair and boat form pyranose & furanose glycosidic bond reducing end disaccharide nomenclature Cellulose ( 1-4 glucose) Amylose ( 1-4 glucose) Amylopectin & Glycogen ( 1-4 & 1-6 glucose) Bacterial Cell Wall Structure (NAG, NAG, tetrapeptide, pentaglycine Conformation of Ring Structures: The most stable conformations are the "chair" forms. The "boat" form is less stable than either chair form. In the case of glucose, the left chair form is more stable than the right chair form. CH2OH CH2OH H OH O OH CH2OH HO OH O OH H OH O O H HO OH OH OH OH OH OH Disaccharides: Linkage of the anomeric carbon of one monosaccharide to the OH of another monosaccharide via a condensation reaction. The bond is termed a glycosidic bond. The end of the disaccharide that contains the free anomeric carbon is referred to as the reducing end because it is capable of reducing various metal ions, such as Cu2+. OH OH OH H O H CH2OH OH O O OH HO O O CH2OH H O H OH O CH2OH CH2OH O OH OH O OH OH OH OH HO OH O H2O Cu2+ O 2H+ + 2e Cuo Nomenclature: To describe disaccharides you need to specify the following: 1. The names of the two monosaccharides. 2. Their cyclic form: O O a) furanose for 5 membered rings b) pyranose for 6 membered rings Furan 3. How they are linked together (one pyran anomeric is always used) 4. The configuration of the anomeric carbon. The six simple rules for naming disaccharides are as follows: 1. The non-reducing end defines the first sugar. 2. Configuration of the anomeric carbon of the 1st sugar (,β) 3. Name of 1st monosaccharide, root name followed by pyranosyl (6-ring) or furanosyl (5-ring) 4. Atoms which are linked together, 1st sugar then 2nd sugar. 5. Configuration of the anomeric carbon of the second sugar (,β) (often omitted if the anomeric carbon is free since α & β forms are in equilibrium.) 6. Name of 2nd monosaccharide, root name followed by pyranose (6-ring) or furanose (5-ring) (If both anomeric carbons are involved, then the name ends in ‘oside”, not ‘ose’) 1 Biochemistry I, Spring Term Lecture 24 March 20, 2013 Lactose (milk sugar): CH2OH O CH2OH O HO CH2OH OH OH O CH2OH O OH OH OH CH2OH O HO O OH OH OH O 3 4 5 CH2OH OH OH OH CH2OH O O OH O OH CH2OH O HO OH OH OH CH2OH CH2OH O HO O O -galactopyranosyl-(1→4)- -glucopyranose 6 CH2OH O HO OH OH (alternate drawing) -galactopyranosyl-(1→4)--glucopyranose Rule # 2 O OH OH OH CH2OH O HO O OH OH OH In solution: OH OH OH OH OH OH -galactopyranosyl-(1→4)-glucopyranose Lactose is the major sugar in mammalian milk. Infants produce lactase to hydrolyze the disaccharide to monosaccharides. Some adults have low levels of lactase. This leads to lactose intolerance. The ingested lactose is not absorbed in the small intestine, but instead is fermented by bacteria in the large intestine, producing uncomfortable volumes of CO2. Sucrose (table sugar): The anomeric carbon of glucose forms a glycosidic bond to the anomeric carbon of fructose. 6 Sucrose CH2OH 5 CH2OH O OH CH2OH O OH glucose 4 OH 3 OH OH O HO OH 1 2 OH 6 CH2OH O CH2OH (alternative drawing) O O 5 glucose fructose HO 2 fructose 4 3 CH2OH 1 -glucopyranosyl-(1-2) -fructofuranoside OH -fructofuranosyl-(2-1) -glucopyranoside C. Polysaccharides: Many monosaccharides linked by glycosidic bonds. Most polysaccharides are polymers of either glucose, or modified glucose. Short-hand nomenclature: In the case of homo-polymers, the short-hand notation is to simply describe the linkage between the glucose units: both the conformation of the anomeric carbon and the carbons participating in the glycosidic bond, i.e. (1-4) instead of β-glucopyranosyl (1-4) β glucopyranose. Energy Storage Polysaccharides CH OH 2 1. Starch [plants] (mixture of amylose and O amylopectin). O amylose = (1-4) glucose. Similar in structure to -helix in proteins: forms a helix with extensive hydrogen bonding. CH2OH O CH2OH CH2OH O O O O O OH CH2OH O OH 2. Amylopectin [plants] = amylose plus (1-6) branches. 3. Glycogen [animals] = more highly branched than amylopectin. CH2OH OH CH2 O O OH OH O OH OH 2 Biochemistry I, Spring Term Lecture 24 March 20, 2013 Release of glucose from glycogen: glucose units released from non-reducing end by the enzyme glycogen phosphorylase, producing glucose-1-phosphate (G-1-P). G-1-P is used to generate energy. CH2OH O CH2OH O O HO CH2OH CH2 CH2OH O O CH2OH O O O HO Inorganic phosphate (Pi) O O O CH2OH O O O O HO CH2 CH2OH O HO O CH2OH O O CH2OH O O CH2OH O O O P O O O O Structural Polysaccharides 1. Cellulose: Structural polysaccharide of plants. 1-4 glucose, can't be digested by mammalian enzymes. Similar in structure to -sheets in proteins: forms flat sheets with multiple hydrogen bonds between strands. Digested by symbiotic microorganisms (such as those in termites) CH2OH O CH2OH O (alternative drawings) OH O OH (1-4) OH O O OH O O CH2OH O O O O OH O CH2OH CH2OH CH2OH CH2OH OH O OH OH OH O OH CH2OH O OH OH O OH O (1-4) OH OH OH 2. Bacterial Cell Walls: Polysaccharide chains of alternating N-acetylglucose amine CH OH (NAG) and N-acetylmuraminc acid (NAM) O OH OH Muramic acid on NAM linked to a small peptide (L-Ala DGln L-Lys D-Ala)- note unusual D-amino acids. OH NH NAM peptide chains are crosslinked with pentaglycine bridges that extend off of terminal Ala and join to L-Lys glucosamine sidechain on adjacent chain, forming a tough crosslinked cell wall. Synthesis of bacterial cell walls is inhibited by antibiotic penicillin. Lysozyme – bactericidal enzyme o hydrolyzes β-(1-4) linkages between NAM & NAG o Transition state is half-chair intermediate. H3C CH2OH CH2OH 2 O OH O O OH O OH OH OH HN OH HN CH3 CH3 2 O O N-acetyl glucosamine (NAG) N-acetylmuramic acid (NAM) NAM OH NAG CH2OH O NH O R1 O O O O CH3 N H O H O NAM O O HN O O O HN O O OH HN HN O Lys Ala NH2 OH CH2OH O R1 O HN O O CH3 O O N O H O CH3 H N H O N CH3 Lys NH2 Ala GLY iso-peptide GLY bond GLY GLY Gln Ala NAG Ala O Ala Lys O OH CH3 O H3C CH2OH Gln HN N Gln HN CH3 O H3C NAM O CH3 O OH O N Ala CH2OH O CH3 O H3C O O O O O CH2OH O CH2OH CH3 O NAG O H3C CH2OH O OH HN O O CH2OH CH3 O CH2OH O Lysozyme N H H2N CH2OH CH3 H O H N O C GLY 3 CH3