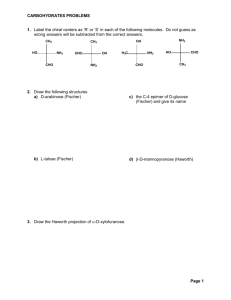
C–OH
advertisement

Carbohydrates and Carbohydrate metabolism (Chemistry of Carbohydrate ) Objective: Understand classification and structure of carbohydrates Understand multistep sequences (pathways) for carbohydrates metabolism. Study the metabolic disorders in carbohydrates metabolism. Carbohydrates and Carbohydrate metabolism (Chemistry of Carbohydrate ) Carbohydrate: They are polyhydroxy aldhydes or ketones or any substances derived from them. OR Compounds that contains at least 3 carbon atoms, a number of OH group, in addition to aldhyde or ketone Formula for simple is (CH2O)n . Carbohydrates Importance and distribution of CHO in animal and plant tissue: Plants: (a) Cellulose: which form the frame work of the plant and has supporting action. (b) Starch: Which is the stored form of CHO. Carbohydrates Classification of CHO: (1) Monosaccharide: They are the simplest units of CHO, cannot hydrolyzed to the simpler form. They can be classified according to either number of carbon atoms, or whether they contain aldhydes or ketons. Carbohydrates (Monosaccharide) (1) according to number of carbons: Trioses:Ex.Glyceraldehydes (aldotriose) Ex. Dihydroxyacetone ( ketotrioses) Tetrosis: Ex. Erythrose (aldotetrosis) Ex. Erythulose (ketotetrosis) Pentoses:Ex. Ribose (aldopentosis) Ex. Ribulose (ketoopentosis) Hexoses: glucose , fructose , galactose, mannose Carbohydrates (Monosaccharide), Stereoisomerism Asymetric carbon atom: A carbon atom that attached to four different atoms or groups of atoms. Any substance containing asymetric carbon atom, it has two different optically active isomers. Isomers: Compounds that have the same chemical formula but have different structure. Ex. Glucose, fructose, mannose, galactose. Monosaccharide, Stereoisomerism Epimers: Compounds that have the same chemical formula but differ in configuration around one carbon atom. Ex. ( D. glucose, D galactose C-4 not galactose and mannose) Enantiomers: A special type of isomers is found in the pairs of structure that are mirror images of each other.( L. glucose, D. glucose) D.glyceraldhyde: in which OH group attached to asymmetric C atom is towards the right. L.glyceraldhyde: in which OH group attached to asymmetric C atom is towards the left. Chemistry of Carbohydrate Stereochemical relations in carbohydrates were explored by Emil Fischer, who also devised a way to represent these molecules. Fischer projection Important Monosaccharides H–C=O H–C=O H–C–OH H–C–OH HO–C–H H–C–OH H–C–OH CH2OH D-Glucose HO–C–H HO–C–H H–C–OH CH2OH D-Galactose H–C=O H–C–OH HO–C–H H–C=O HO–C–H HO–C–H H–C–OH H–C–OH H–C–OH H–C–OH CH2OH CH2OH D-Mannose D-Fructose If only one of several stereocentres in a molecule is diferent, such isomers are epimers. H–C=O H–C–OH HO–C–H H–C–OH H–C–OH CH2OH In Fischer projection: Chiral C farthest away from highest oxidized C has OH to right D-sugar. b a In Haworth projection: if C1-OH and C5-CH2OH on same side of ring = b, if on different sides = a (anders, alternative) Cyclization of monosaccharide The simple chain formula fails to explain the many reaction so, Less than 1% of each of monosaccharides are found in a ring form, in which the aldhyde or keton group has reacted with an alcohol group in the same sugar. Formation of the ring results in the creation of anomeric carbon atomat C-1 of an aldose and on C-2 of a ketose These structure are designated the α & β configuration of the sugar. If the remaining OH is on the right α- sugar If the remaining OH is on the left β - sugar Cyclization of monosaccharide Mutarotation: The cyclic α & β anomers of sugar are in the equilibrium with each other, and can be spontaneously inter-converted in a process called mutarotation. Representation of sugar conformation: (1)Fisher projection. (2)Haworth projection Cyclization of monosaccharide H–C=O b H–C–OH HO–C–H H–C–OH a H–C–OH CH2OH D-glucose open chain Fischer projection D-glucose ring form a-D-glucose Haworth projection a-D-glucose Chair conformation anomeric C Chemistry of Carbohydrate Reducing sugar: If the O2 of anomeric Catom is not attached to any other compound, that sugar is a reducing sugar A reducing sugar can react with the chemical reagent (Ex. Bendict solution& fehling solution) and reduce the reactive compound, with the anomeric C- atom is oxidized. Important Monosaccharides Disaccharide & polysaccharide Disaccharides: These are formed by condensation of 2 molecules of monosaccharide by a glycosidic linkage. oligosaccharides: contain from3 to about 12 of monosaccharide units. polysaccharides: contain more than 12 of monosaccharide units. Disaccharides Lactose Gal-b-1-4-Glu Maltose Glu-a-1-4-Glu Sucrose Glu-a-1-b-1-Fru polysaccharide Starch: It is the most important polysacharide. It is a polyglucose, a-1-4 linked. Ther are two main components: amylose – linear, ca. 500 – 20 000 linked glucose units amylopectin – branched through a-1-6 bonds every ~25 AGU glycogen : body polysachharide: similar to amylopectin, higher branched Cellulose: is composed of b-1-4 linked glucose units. This bond cannot be cleaved by our digestive enzymes. Important part of cell walls and dietary fibre. Polysaccharides – Starch, glycogen a-1-6 branch point in glycogen Polysaccharides - Cellulose Stability of cellulose is increased through formation of crystalline regions with extensive hydrogen bonding Complex carbohydrates CHO can also attached by glycosidic bonds to nonCHO structure (a glycone) (Ex. Purine and pyrimidine as in nucleic acids, aromatic ring as those found in steroid & bilirubin, proteins as glycoproteins& glycosaminoglycans, and lipids as in glycolipids) to form glycosides. O- and N- glycosides: If the group on the non-CHO is an OH group, the structure is an O- glycosides, whereas If the group on the non-CHO is an NH2 group, the structure is an N- glycosides. The Aldoses C3 – C6 CHO ET RAXL All Altruists Gladly Make Gum In Gallon Tanks The Ketoses C3 – C6 Dihydroxyacetone Related Aldose + ending “ulose“ Related Aldose + ending “ulose“ PFST Glucose structure in solution Pentoses and hexoses can adopt two ring structures: 5-membered (Furanoses, after furan), and 6-membered (Pyranoses, after pyran). Glucose is in equilibrium between two pyranose forms. At equilibrium, there is ca. 65 % b-D-Glucopyranose, ca. 35 % a-D-Glucopyranose, and <1 % of the open-chain form. O ~ 35 % Furan O ~ 65 % Pyran <1% Fructose structure in solution Fructose adopts a furanose structure, preferring the a-anomer. Other roles and modifications of carbohydrates In addition to their role as fuel molecules, carbohydrates are important molecules as: - building blocks of nucleic acids - antigens (blood groups, cellular interaction through glycosylated surface proteins) - glycosylation of proteins quality control system for protein folding - glycosylation also determines functional properties of proteins - metabolic intermediates and specialised molecules