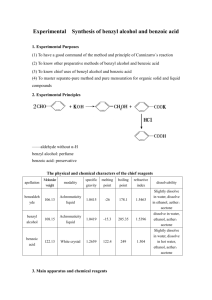
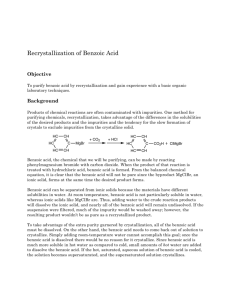
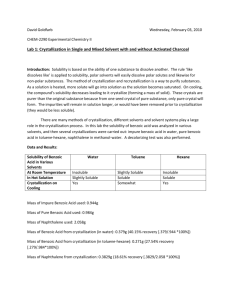
Benzoic Acid Purification: Recrystallization vs. Extraction
advertisement
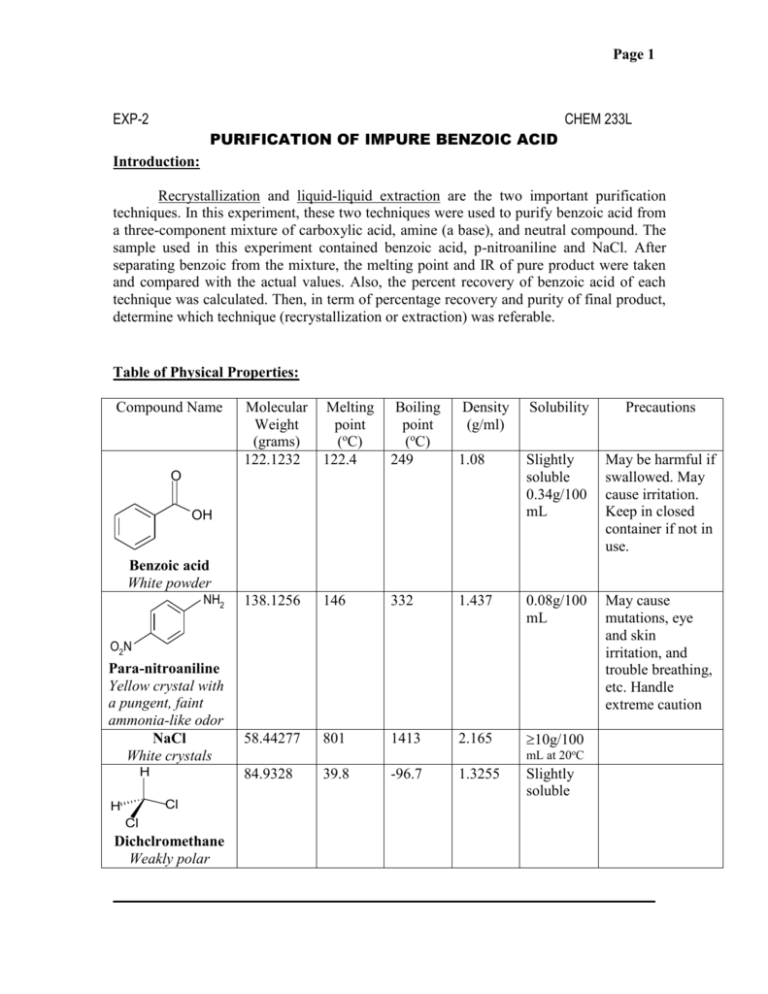
Page 1 EXP-2 CHEM 233L PURIFICATION OF IMPURE BENZOIC ACID Introduction: Recrystallization and liquid-liquid extraction are the two important purification techniques. In this experiment, these two techniques were used to purify benzoic acid from a three-component mixture of carboxylic acid, amine (a base), and neutral compound. The sample used in this experiment contained benzoic acid, p-nitroaniline and NaCl. After separating benzoic from the mixture, the melting point and IR of pure product were taken and compared with the actual values. Also, the percent recovery of benzoic acid of each technique was calculated. Then, in term of percentage recovery and purity of final product, determine which technique (recrystallization or extraction) was referable. Table of Physical Properties: Compound Name Molecular Weight (grams) 122.1232 Melting point (oC) 122.4 Boiling point (oC) 249 Density (g/ml) Solubility Precautions 1.08 Slightly soluble 0.34g/100 mL May be harmful if swallowed. May cause irritation. Keep in closed container if not in use. 138.1256 146 332 1.437 0.08g/100 mL May cause mutations, eye and skin irritation, and trouble breathing, etc. Handle extreme caution 58.44277 801 1413 2.165 10g/100 O OH Benzoic acid White powder NH2 O2N Para-nitroaniline Yellow crystal with a pungent, faint ammonia-like odor NaCl White crystals H mL at 20oC 84.9328 39.8 -96.7 1.3255 Slightly soluble Cl H Cl Dichclromethane Weakly polar ________________________________________________________________________ Page 2 Safety Notes: Benzoic acid may be harmful and cause irritation. Keep it in closed container if not in use. Para-nitroaniline may cause mutations, eyes and skin irritation, and trouble breathing Handle it extreme caution Procedure: EXP 2A: RECRYSTALLZATION OF IMPURE BENZOIC ACID FROM WATER 1. Heat the hot plate to 110-130 oC. Place 100 mL of water in a 250 mL beaker and bring to a boil. 2. Place approximately 1.0 g of impure benzoic acid in a 125 ml flask and add approximately 25 mL of hot water. If your sample appears to have an unwanted color, it can be removed later with charcoal. 3. Heat the mixture on a hot plate until the water boils. If necessary, add small quantities of hot water with a Pasteur pipet until all the white benzoic acid was dissolved into solution. If you see particles that do not seem to dissolve even with additional hot water, don’t worry; they will be removed in the filtration step. 4. Remove the flask from the hot plate and allow to cool down about 10 oC before adding decolorizing charcoal. Decolorizing charcoal is usually added to remove colored impurities. The amount of the charcoal added depends on the size of your material. Between 5-10% of the weight is typical amount. 5. Place the solution containing the charcoal back on the hot plate and boil for 5 minutes. 6. Remove any solid insoluble impurity by using the technique of gravity filtration. The hot benzoic acid was filtered using a short stem funnel and a 125mL flask to collect the filtrate. Notice that the filtrate should be kept hot to prevent early crystallization. Keep both flasks on the hot plate to keep hot at all times. The funnel should not overflow to prevent contaminating your filtrate with charcoal. If this occurs, the hot filtration will be repeated a second time. 7. Chill the filtered benzoic acid solution in an ice bath. Then filter the crystals on a Buchner funnel and wash the flask and crystals with a small amount of ice-cold water (in separate beaker, equal parts ice and water were mixed, about 25 mL each). 8. Next, dry the crystals on the funnel by using the vacuum to pull air over them for at least 5 minutes. If the crystals still have a yellow impurity, recrystallize the crystals from a minimum amount of hot water once again. It is not necessary to use charcoal. 9. Dry the crystals overnight. 10. The melting point and IR was determined on next day of class. EXP-2B: PURIFICATION OF IMPURE BENZOIC ACID BY EXTRACTION: Page 3 1. Weigh 500 mg of the chemical mixture containing benzoic acid, p-nitroaniline and NaCl. 2. Add about 5 mL of water to a separatory funnel (make certain the stopcork works properly, is tight but able to be turned, and that is does not leak). 3. Add about 15 mL of CH2Cl2 . Two phases are observed. Even though the liquids are each colorless, dichloromethane, which is denser than water, will go to the bottom. The dividing line between two liquids should be seen. 4. Add the mixture to the liquid contents of the funnel. Insert a ground-glass stopper into the top of separatory funnel and mix well. You should be able to observe that all of the crystals dissolve and disappear from view. At this time, the NaCl has dissolved into the water, making an aqueous salt solution. The benzoic acid and pnitroaniline was dissolved in CH2Cl2. 5. Extract the CH2Cl2 twice with 20 mL of water in order to remove the NaCl. To extract effectively, shake the separatory funnel to thoroughly mix the two phases. 6. Periodically, with the stopper firmly in place (keep a finger on the stopper at all times), turn the separatory funnel upside down (with the tip near the stopcock pointing upward). 7. Open the stopcock to relieve any pressure (you will probably hear a hiss or sound of escaping gas. The water phase will be found on top. The organic phase is at the bottom. 8. Collect the organic phase in a 125 mL flask and collect the aqueous phase in a beaker. Do not discard any of the solution until you have completed the experiment. 9. Return the organic phase into the separatory funnel. 10. Add about 10 mL of the 10% NaOH solution to make the upper aqueous phase basic (excess OH- ion; turn red litmus blue) and neutralize the benzoic acid (which was dissolved in the organic phase) and the benzoate ion was converted, which is charged and now will partition into the aqueous phase. 11. Mix the content of the separatory funnel for a couple minutes and vent 3-4 times; the aqueous phase from the upper organic phase should be ready. 12. Place the separatory funnel in the O-ring clamp and let the two phases separate completely. When the interface is apparent, remove the ground-glass stopper and open the stopcock gently. 13. Collect the aqueous sodium benzoate in a 125 mL flask. 14. Add enough 6 M HCl to the aqueous phase to react with the sodium benzoate to convert it back into benzoic acid, which is mostly insoluble in water. 15. When enough acid was added, test the liquid by the blue litmus paper to verify acidity (blue litmus paper turns red) 16. When the liquid was known to be slightly acidic, collect the recrystallized benzoic acid. Reference: www.chemfinder.com http://ptcl.chem.ox.ac.uk/MSDS EXP-2B: PURIFICATION OF IMPURE BENZOIC ACID BY EXTRACTION: Questions: Answer the following questions. Page 4 1) Two techniques were used in this experiment. a. Recrystalization b. Extraction.