File
advertisement

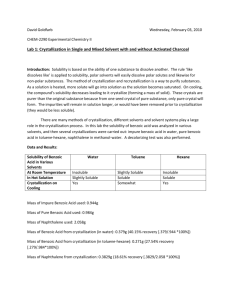
INTERMOLECULAR FORCES 1. Van der Waals Forces or London Dispersion Force Weak force. Brought about by the fluctuating charge of the molecule due to electron distribution. This force results to temporary dipole, causing induced polarization. An increase in the number of carbon causes an increase Van der Waals Force. Branching causes a decrease of Van der Waals Force. 2. Dipole-Dipole Observed among partly ionic or polar molecules. Brought about by the interaction of two charges at both ends – one is slightly positive and the other is slightly negative. It induces polarization causing a positive charge to be negative. o Partially negative elements: Halogens, oxygen o Partially positive element: Carbon 3. Hydrogen Bonding Strongest type of intermolecular force Can either be intramolecular or intermolecular o Intermolecular H-bonding raises the boiling point of organic compounds. o Intramolecular H-bonding lowers the boiling point because of the decreased tendency for interaction with other molecules. Involves an interaction between a polarized OH bond and NH bond. Happens when two molecules that contain a hydrogen atom bonded to a very electronegative atom. Type of Force Relative Strength Exhibited by Van der Waals Weak All molecules Dipole-Dipole Moderate Molecules with net dipole Molecules with an O-H, NH, or H-F bond Hydrogen Bonding Strong Example CH4 H2O H2O H2O Experiment 8: Boiling Point It is the temperature at which the vapor pressure of the substance is equals the pressure of the atmosphere above it. It is a physical constant that can be used in identification and characterization, as well as a criterion of purity of a substance. Pure compounds have constant boiling point. Mixtures have a boiling point range except for azeotropes. Compounds involving ionic bonds have higher boiling point because the amount of heat required to separate the ions is higher than the amount required to separate molecules in covalent compounds. Presence of impurities may affect the boiling point of a liquid. Non-volatile impurities usually increase the boiling point of the liquid due to a decrease in the vapor pressure. Volatile impurities usually decrease the boiling point of the liquid. Test Compound: n-Butyl Alcohol Boiling Point: 118°C Test Compound: n-amyl Alcohol Boiling Point: 136-138°C Test Compound: tert-Butyl Alcohol Boiling Point: 82°C N-butyl alcohol vs n-amyl alcohol n-amyl alcohol has more carbon, thus, has stronger Van der Waals force. N-butyl alcohol vs tert-butyl alcohol tert-butyl alcohol has lower boiling point because of the branching. Experiment 9: Melting Point It is the temperature at which the solid becomes a liquid. Solids with greater cohesive forces exhibit higher melting points. Pure crystalline solids normally have sharp melting points. Test Compound: Stearic acid Melting Point: 68-70°C - long straight chain; Van der Waals Test Compound: Benzoic acid Melting Point: 120-122°C Benzoic acid - (carboxyl group) OH makes the benzoic acid more open for h-bonding. - More h-bonds means more difficult to melt. Test Compound: Salicylic acid Melting Point: 155-160°C Salicylic has carboxylic acid group thus it has more intermolecular forces making it more difficult to melt. Experiment 10: Solubility Test Compound: OH n-Propyl Alcohol n-Propyl alcohol + Water n-Propyl alcohol + Ether soluble soluble Solubility Class: S1 (water soluble) Structural Effect: - Hydroxyl group easily forms H-bonds with water. - The length of the carbon chain also implies weak Van der Waals force. Test Compound: Naphthalene Naphthalene + Water insoluble Naphthalene + NaOH insoluble Naphthalene + HCl insoluble Naphthalene + H2SO4 insoluble Solubility Class: INERT Structural Effect: - The fused benzene ring makes the compound resonance stabilize. Test Compound: Urea Urea + Water soluble Urea + Ether insoluble O C H2N NH2 Solubility Class: S2 (water soluble) Structural Effect: - Soluble in water because of the NH group. - The double bond could also form H-bonds. - This is an example of an exemption to “like dissolve like.” Test Compound: Aniline NH2 Aniline + Water insoluble Aniline + NaOH insoluble Aniline + HCl soluble C6H5NH2 + HCl C6H5N+H3ClSolubility Class: BASIC Structural Effect: - NH site reacts with Cl. Test Compound: Benzoic Acid O OH Benzoic acid + Water insoluble Benzoic acid + NaOH soluble C6H5COOH + NaOH C6H5COO-Na+ + H2O Benzoic acid + NaHCO3 partially soluble C6H5COOH + NaHCO3 C6H5COO-Na+ + H2O + CO2 Solubility Class: A1 (acidic) Structural Effect: - COOH group is drawn to the benzene ring. - The hydroxyl group in H2O cannot interact with any of the groups in benzoic acid. The more the intramolecular H-bonding the more insoluble the sample is. Polar solvents dissolve the solute because of dipole-dipole interaction. Non-polar solvents dissolve the solute because of dipole-dipole interaction. Experiment 11: Acidity and Basicity Arrhenius acid – a substance that produces hydrogen ions H+ or hydronium ions in water. Arrhenius acids are frequently referred to as proton donors, hydrogen ion donors, or hydronium ion donors Chemical formula is written with the acidic hydrogen first. o Example: HCl Arrhenius base – a substance that produces hydroxide ions OH- in water. NaOH is an example of an Arrhenius base. Bronsted-Lowry acid – a substance that donates a proton (H+). Bronsted-Lowry base – a substance that accepts a proton (H+). Arrhennius Bronsted-Lowry Lewis Acid Ionize to give H+ A proton donor An electron pair acceptor Base Ionize to give OHA proton acceptor An electron pair donor