Periodic Trends Data Analysis
advertisement

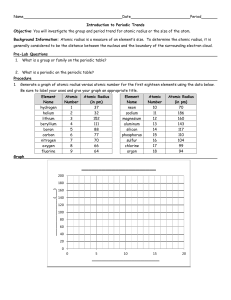
Periodic Trends Finalizing our Understanding of the Organization of the Periodic table Directions: Define the following terms. Atomic Radius - Ionization Energy - Electronegativity - Directions: Using Graphs to Visualize Trends on the Periodic Table On the next page you will find 6 different tables. On a separate piece of paper, create a graph for each table, graphing atomic number versus the trend of interest. Be sure to title your graphs, and label the x-axis and y-axis. Once finished check graphs with Ms. Herndon before moving on to the questions below Electronegativity Across a Period (Row) Element Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Atomic Number Electronegativity 3 1.0 4 1.5 5 2.0 6 2.5 7 3.0 8 3.5 9 4.0 Electronegativity Down a Group (Column) Element Beryllium Magnesium Calcium Strontium Barium Radium Atomic Number 4 12 20 38 56 88 Electronegativity 1.5 1.2 1.0 1.0 0.9 0.9 Atomic Radius Across a Period (Row) Element Atomic Number Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 3 4 5 6 7 8 9 10 Atomic Radius (10-10 m) 2.1 1.4 1.2 0.91 0.75 0.65 0.57 0.51 Atomic Radius Down a Group (Column) Element Beryllium Magnesium Calcium Strontium Barium Atomic Number 4 12 20 38 56 Atomic Radius (10-10 m) 1.4 1.7 2.2 2.5 2.8 Periodic Trends Finalizing our Understanding of the Organization of the Periodic table Ionization Energy Across a Period (Row) Element Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Atomic Number Ionization Energy (J/mol) 3 519 4 900 5 799 6 1088 7 1401 8 1036 9 1682 10 2076 Ionization Energy Down a Group (Column) Element Beryllium Magnesium Calcium Strontium Barium Radium Atomic Number 4 12 20 38 56 88 Ionization Energy (J/mol) 900 736 590 548 502 510 Directions: Using your graphs you just drew for the above data tables, answer the following questions. 1) Does electronegativity increase or decrease left to right across a period? 2) Does electronegativity increase or decrease down a group? 3) Does ionization energy increase or decrease left to right across a period? 4) Does ionization energy increase or decrease down a group? 5) Does atomic radius increase of decrease left to right across a period? 6) Does atomic radius increase or decrease down a group?