Chapter 15
advertisement

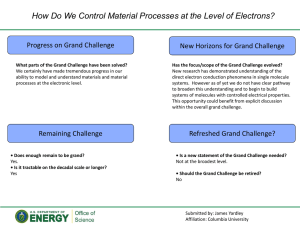
Chapter 15 Critical Thinking Exercise Chemical Kinetics Oxygen in the air we breathe is in its "ground" (not energetically excited) state and is symbolized by the abbreviation 3O2. It is a free radical-in fact it is a diradical, as it has two unpaired electrons. Electrons "spin" (that is, rotate about an axis passing through the electron). Molecules whose outermost pair of electrons have parallel spins (symbolized by ) are in the "triplet" state; molecules whose outermost pair of electrons have antiparallel spins (symbolized by ) are in the "singlet" state. Ground-state oxygen is in the triplet state (indicated by the superscripted "3" in 3O2) - its two unpaired electrons have parallel spins, a characteristic that, according to rules of physical chemistry, does not allow them to react with most molecules. Thus, ground-state or triplet oxygen is not very reactive. However, triplet oxygen can be activated by the addition of energy (94.1 kcal/mole) and transformed into reactive oxygen species. If triplet oxygen absorbs sufficient energy to reverse the spin of one of its unpaired electrons, it forms the singlet state. Singlet oxygen, abbreviated 1O2*, has a pair of electrons with opposite spins; though not a free radical it is highly reactive. (The * symbol is used to indicate that this is an excited state with excess energy.) It should be emphasized that neither triplet- nor singletstate molecules are necessarily in the excited state; the designation of "triplet" or "singlet" refers only to the spin state. The MO diagrams for both species are given below. Normal aerobic metabolism produces as its by-products various highly reactive molecules, collectively termed "oxidants". These oxidants include a variety of electron-stealing molecules known as free radicals, as well as the highly reactive singlet form of oxygen. Some of these reactive Chapter 15 molecules (e. g., superoxide, hydrogen peroxide, and nitric oxide) are physiologically useful and, in fact, are necessary for life, but can also be harmful if present in excess or in inappropriate situations. All of these oxidants can react with various components of a living cell, such as proteins, DNA, or lipids (fats), thus causing damage by changing the chemical structure of these components. Such damage has been linked to a number of pathological conditions including aging, atherogenesis , age-related macular degeneration , and carcinogenesis . For us, oxygen is therefore both necessary and harmful; this sobering conclusion has been referred to as the "paradox of aerobic life." The human body has evolved a large array of endogenous antioxidant defenses against oxidative stress, including antioxidant enzymes such as superoxide dismutase, catalase, and various peroxidases. However, these endogenous antioxidants do not completely protect against the sum of oxidative stresses challenging the body, and thus there is net oxidative damage that in the long term contributes to aging and various diseases. In addition to the body's endogenous defenses against oxidative stress, diet-derived antioxidants--including ascorbic acid (Vitamin C), alphatocopherol (Vitamin E), and the carotenoids--may be important in protecting against disease and age-related phenomena. Diet-derived antioxidants may be classified on the basis of their solubility as either lipid-soluble (i. e., soluble in fats), or water-soluble. Lipid-soluble antioxidants include vitamin E and the carotenoids, while vitamin C is a common water-soluble antioxidant. -http://www.astaxanthin.org/ 1. The structures of vitamins C and E are given below. Which is vitamin E? a. b. 2. How many chiral carbons are in vitamin E? a. 0 b. 1 c. 2 d. 3 e. 4 3. Build the model of vitamin C. Is the ring C in the R or S chiral configuration? a. R b. S 4. Is the formation of singlet oxygen an exo- or endothermic process? a. exothermic b. endothermic 5. Using the information at the end of the questions, a carotenoid is in what state after quenching singlet oxygen? a. singlet b. triplet c. ground d. excited e. both b and d 6. Looking at the units on the rate constant for singlet oxygen quenching by a carotenoid given in the table, what order is the reaction? a. zero b. first c. second d. third 2 Chapter 15 7. What is the rate law? a. rate = k b. rate = k[1O2*] c. rate = k[1Car] d. rate = k[1O2*][1Car] e. rate = k[1O2*][1Car]2 8. Which of the carotenoids listed has the highest quenching rate? a. -carotene b. lycopene c. astaxanthin d. zeaxanthin e. lutein 9. The quenching capability of a carotenoid appears to be related to a. its molecular mass b. the number of carbon atoms c. the number of double bonds d. the number of conjugated double bonds e. the number of O atoms 10. In the two-step mechanism for singlet oxygen quenching, which is the slow step? a. first b. second 3 Chapter 15 4 Chapter 15 5