Supplementary Methodologies (docx 32K)
advertisement

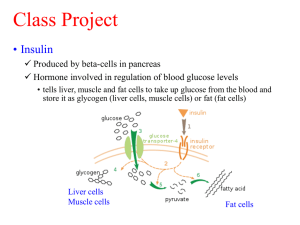
Supplementary Methodologies: Materials: Normal pellet diet (NPD; 10 % calories from fat) was procured from Aashirwad industries, Mohali, Punjab, India. Lard was procured from local slaughter house. High fat diet (HFD; 60% calories from fat) was prepared using a standard composition described elsewhere.1 Energy output of NPD is ~ 4.75 kcal/gm whereas HFD had caloric values of 5.25 kcal/gm. The diets fed to animals (HFD to HFD + IMOs/ Lycopene/ combination of both) were of isocaloric in nature. LycoVit®10 CWD/S containing 10 % Lycopene as was a kind gift from BASF-the chemical company (BASF India Ltd, Mumbai, India). Isomaltooligosaccharides (IMOs) as VitaFiberTM Basic (Powder), was a kind gift from BioNeutra North America Inc. It is freely soluble in water, sweetness ~ 60 % of sucrose, low calories, non-carcinogenic, ≥ 90% non digestible/ resistant oligos, ≤3% free glucose, free of glutan and other allergen, stable to wide range of pH and heat condition, least flatulent, pleasant mouth feel and humectants. All the reagents used were of highest commercial grades available. Doses and anthropometric paramteres: Lycopene and IMOs were dissolved in distilled water and administered par orally and the control groups received distilled water (five days in week i.e. except Sunday and Wednesday). Doses of lycopene and IMOs selected for the study were based on available literature.2-6 For the dose preparation of lycopene 10% active lycopene in the powder was considered for calculation. The study was conducted for a total of 12 weeks. Body weights were measured weekly. Naso-anal length and waist circumference was measured in the last week of the study. The anthropometric parameters e.g. BMI (body weight in g/ naso-anal length2 in cm), Lee’s index (body weight in g0.33×1000)/ naso-anal length in cm). In the last week total locomotor activity was measured using photoactometer (IMCORP), Ambala, India. At the end of the experiment, from the fasted mice, blood was collected retroorbitally and mice were sacrificed by cervical dislocation, except the animals were kept for histological examinations. Visceral white adipose tissue (vWAT), subcutaneous white adipose tissue (sWAT), brown adipose tissue (BAT), hypothalamus, pancreas, liver were dissected, snap frozen and stored at -80 °C. Entire gut from the base of oesophagus to rectum was isolated and weighed. The cecum was dissected and its content was isolated asceptically, weighed and snap frozen for extraction of bacterial DNA. At the same time content of alimentary canal was removed and disembowelled body weight was calculated by subtracting weight of gut to total body weight. Blood and tissue biochemistry: The OGTT test was performed during the 12th week (4th and 5th) day before the sacrifice of the mice. Mice were fasted overnight (12 h), and basal blood glucose concentration (0 min) was determined using Glucocard TM strips (Arkray Factory Inc., Japan). Then 2 g/kg body weight of glucose was administered orally and blood glucose (mg/dL) was determined at 15, 30, 60, 120 minutes. Using the basal blood glucose level (mM/L) and fasted serum insulin level, the % beta cell activity (%B) and insulin resistance (IR) was calculated using updated Homeostasis Model Assessment (HOMA) calculator 7 (HOMA2 calculator v2.2.3 developed by Diabetes Trial Unit, University of Oxford). Another insulin sensitivity index quantitative insulin sensitivity check index (QUICKI) [QUICKI = 1/(log glucose in mg/dL + log insulin in µIU/ mL)] and McAuley’s index in terms of fat-free mass (Mffm/I) for insulin resistance [Mffm/I = e(2.63 – 0.28 ln (Insulin in µIU/mL) – 0.31 ln (TG in mM/L)] using fasted blood glucose and serum insulin and serum TG were calculated.8 Serum collected at the end of experiment was used for determining serum lipid profile i.e. total cholesterol, low density cholesterol (LDL-c), high density cholesterol (HDL-c), triglyceride using commercially available kits (Accurex biomedical pvt. ltd., Maharashtra, India) and free fatty acid (Sigma-aldrich Inc., St. Louise, MO, USA). Liver lipids were extracted using method of Folch et al., 1957.9 The extracted lipids were resuspended in 2% Triton X-100 10 and similarly assayed for total cholesterol, LDL-c, HDL-c and triglyceride (TG) using commercial kits as for serum. Atherogenic fraction in liver was calculated as TC minus HDLc. Different biochemical parameters e.g. lipid peroxidation (LPO) in terms of malondialdehyde (MDA), reduced glutathione (GSH), total nitrite, superoxide dismutase (SOD) and catalase were assayed in supernatant of tissue homogenate (10 % w/v) of sWAT, liver, pancreas, vWAT. Only total nitrite was assayed in BAT tissue. All these estimation were performed using previously described methods 11 and the values were expressed in terms of per mg protein, which is quantified using Bradford’s reagent. Gene expression analysis: Total RNA was isolated from Liver, vWAT and hypothalamus using the Trizol® based RiboPure RNA extraction kit (InvitrogenTM) according to manufacturers’ instructions. Total RNA was quantified using Infinite® M200 Pro NanoQuant (Tecan) and the integrity and purity was determined using 1% agarose gel. Intact RNA samples [1µg (vWAT), 2 µg (liver) and 3 µg (hypothalamus)] were used for c-DNA synthesis using ReverTra Ace® qPCR RT master mix with gDNA remover kit (Toyobo c. ltd., Japan), High capacity RNA-to-cDNA kit (Applied Biosystems) and SuperScript III First-strand synthesis supermix kit (InvitrogenTM) respectively according to manufacturer’s instructions. Quantitative PCR: The relative expression of different genes in Liver and vWAT (suppl. table 1 - for the list of genes and primers) were determined using SYBR® based dye using quantitative PCR (Applied Biosystem 7500 Fast Real-Time PCR machine). Anorectic and orexigenic gene expression in hypothalamus was determined using a customized mouse PCR array plate (CAPM12379C, SABiosciences, Qiagen). RT-PCR was carried out under the following conditions: 95ºC for 10 min, followed by 45 cycles of 95ºC and 60ºC for 1 min. Data were analysed using the ∆∆Ct method, and values are expressed as fold change relative to the NPD group. Histological Examinations: Animals were perfusion fixed by 10% buffered formalin and the liver, vWAT, ileum and colon were isolated and stored in 10 times volume of 10% buffered formalin for further processing using haematoxylin and eosin staining (H&E) and all the images were captured using 20X objective (Final camera magnification 14X). Only frozen tissues were used for neutral lipid specific oil red O (ORO) staining. For H&E staining paraffin embedded tissues were microtomized (3 – 5 µm) and stained. The mean adipocyte size in adipose tissue sections were estimated in 20 images of each tissue (2 animals per group), using Imagescope (Version 12.1.0.5029, Aperio Technologies Inc.) by manual counting. The cell size were categorised on a 10 point arbitrary scale: Category 1 = 240 – 499 µm2; 2 = 500 – 999 µm2; 3 = 1000 – 1499 µm2; 4 = 1500 – 1999 µm2; 5 = 2000 – 2499 µm2; 6 = 2500 – 2999 µm2; 7 = 3000 – 3499 µm2; 8 = 3500 – 3999 µm2; 9 = 4000 – 4499 µm2; 10 = 4500 µm2 <. Ileal sections were also analysed by same software for villus and crypts length. Frozen liver tissues (0.8 cm × 0.8 cm) were directly embedded in tissue freezing medium (Leica Biosystem Nussloch GmbH, Germany) and 12 µm sections were made for staining with ORO dye. Images (2 tissues per group, 10 representative images per tissue, 40X objective) were analyzed using ImageJ software as described elsewhere.12 For quantification of entrapped ORO, sections were washed with 200 µL of isopropyl alcohol and washed liquid were collected and absorbance was determined at 510 nm. The relative absorbance of extracted ORO was calculated as % of NPD group. Microbial analysis of cecal content: Approximately 100 mg of cecal content was taken and DNA was isolated using QIAmp® DNA stool mini kit (Qiagen) according to manufacturer’s instruction. Quantification was performed using Infinite® M200 Pro NanoQuant (Tecan), integrity was evaluated on 0.8% agarose gel. qPCR was performed for Lactobacillus spp. (LAB), Bifidobacteria (BIF), Akkermensia muciniphilia (AKK), Roseburia spp. (ROS), Enterobacteriaceae (ENTB), Bacteroidetes (BACT), Firmicutes (FIRM), taking total bacteria (TB) as internal control was performed as described above (suppl. table 1 – For list of genes and primers). Short chain fatty acids (SCFAs) analysis in cecal content: The system was equipped with Agilent’s controller pump (model no. G1311C), an auto sampler unit (model no. G1329B) and a diode array detector (DAD; model no. G1315D) set at 210 nm (peak width 2.5 Hz, band width 2nm). Chromatographic separation of acetate, propionate and butyrate was achieved at 60ºC using an anion exchange column (300 × 7.7 mm; 8 µm particle size) along with a guard column made with the same material. The mobile phase a mixture of 0.1% formic acid in Milli-Q water (Merck Millipore, 0.22 µm filtered, resistivity 18.1 – 18.3 MΩ cm) was used. The column was equilibrated and eluted with isocratic flow rate of 0.6 mL/min at 60ºC for 30 min. Data was acquired and processed with EZchrom elite software with the system suitability option installed. The method was optimized and validated in house (unpublished data). Weighed samples were suspended in sufficient volume of acidified water (pH 1 – 2) by rigorous vortexing. Then keeping at room temperature (10 min) followed by centrifugation (6000 rpm, 20 min, 4ºC) and filtration with 0.2 µm nylon filter (Millipore Millex-GN). Isobutyric acid was used as an internal standard (5µM). Injection volume was set at 20 µL and minimum two technical and three biological replicates were processed in each group analyzed. Data was represented as µM concentration of these SCFAs per mg sample used. REFERNCES: 1. Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005; 52:313-20. 2. Kohmoto T, Fukui F, Takaku H, Mitsuoka T. Dose-response Test of Isomaltooligosaccharides for Increasing Fecal Bifidobacteria. Agricultural and Biological Chemistry 1991; 55:2157-9. 3. Oku T, Nakamura S. Comparison of digestibility and breath hydrogen gas excretion of fructo-oligosaccharide, galactosyl-sucrose, and isomalto-oligosaccharide in healthy human subjects. Eur J Clin Nutr 2003; 57:1150-6. 4. Kaneko T, Kohmoto T, Kikuchi H, Shiota M, Iino H, Mitsuoka T. Effects of Isomaltooligosaccharides with Different Degrees of Polymerization on Human Fecal Bifidobacteria. Bioscience, biotechnology, and biochemistry 1994; 58:2288-90. 5. Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol 2009; 47:2522-30. 6. Kuhad A, Sharma S, Chopra K. Lycopene attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pain 2008; 12:624-32. 7. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21:2191-2. 8. Oterdoom LH, de Vries AP, van Son WJ, van der Heide JJ, Ploeg RJ, Gansevoort RT, et al. Validation of insulin resistance indexes in a stable renal transplant population. Diabetes Care 2005; 28:2424-9. 9. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry 1957; 226:497-509. 10. Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem 2003; 278:51664-72. 11. Singh DP, Chopra K. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacology Biochemistry and Behavior 2014; 120:33-42. 12. Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protocols 2013; 8:1149-54.