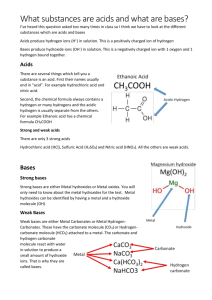
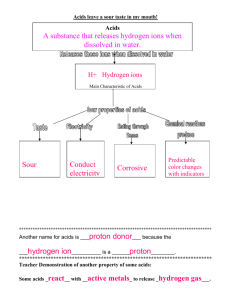
8-2 Acids and Bases Objectives: Students will recognize some acids
advertisement

8-2 Acids and Bases Objectives: 1. Students will recognize some acids and bases as household chemical compounds thatare not necessarily strong or dangerous. 2. Students will realize that acids and bases are not necessarily strong or dangerous. 3. Students will determine the pH of different chemical compounds and categorize them as acids or bases. 4. Students will investigate how the difference between acids and bases correlates to the difference in hydrogen ion concentration of solutions of the two classes of compounds. 5. Students will investigate the concept of solute concentration. 6. Students will dilute an acid to prepare more dilute concentrations of hydrogen ions. 7. Students will measure the change in pH when an acid is diluted. 8. Students will correlate a low pH with a high hydrogen ion concentration and a high pH with a low hydrogen ion concentration. 9. Students will perform a neutralization reaction by mixing an acid and a base. 10. Students will determine the extent of neutralization reaction by measuring the pH of the reaction. 11. Students will observe that in a neutralization reaction, a base decreases the concentration hydrogen ions resulting in an increase in the pH. Focus Questions: What properties make acids and bases chemically reactive? What is the relationship between the concentration of hydrogen ions (H+) in a solution and the chemical reactivity of strong and weak acids? What is the relationship between the concentration of hydrogen ions (H+) in a solution and the chemical reactivity of strong and weak bases? What is the relationship between the pH of a solution and the concentration of hydrogen ions (H+)? How can the concentration of hydrogen ions (H+) be changed? What happens to the concentration of hydrogen ions (H+) when an acid and a base react?