Honors Chemistry Test Topics: Chapters 4 & 5

HONORS CHEMISTRY
CHAPTER 4 AND 5 TEST TOPICS
Test Date: December 11, 2014
Chapter 4:
Know the following scientists and their contributions to the evolution of the atomic model. o Democritus, Dalton, Thomson, Rutherford, Chadwick
Know experimental set-up, observations, and conclusions based on evidence for each scientist and their experiments bolded above.
Be able to draw and label the model of the atom for each scientist mentioned above.
Know Dalton’s postulates.
Compare and contrast between scientists listed above.
Distinguish atoms based upon their number of subatomic particles.
Distinguish between isotopes.
Apply the average atomic mass equation to solve isotope problems.
Chapter 5:
Describe Bohr’s contribution to the atomic model and his ideas and his analogy on energy levels. o Understand why Bohr’s experiment failed for more than 1e- systems.
Be able to describe Schrödinger and Heisenberg’s contributions to the atomic model and how the quantum mechanical model came to be accepted. o Electron Cloud vs. Fixed Orbits
Quantum Numbers o Know the range for each of the 4 Quantum numbers. o Be able to analyze a quantum set for its validity.
Atomic Orbitals o Understand the concept of sublevels vs. atomic orbitals and their electron occupancies.
Electron Configurations o Identify and apply the three rules that govern electron filling patterns to form electron configurations. o Be able to provide an electron configuration for any element on the periodic table using multiple notations.
Be able to draw the highest occupied sublevel
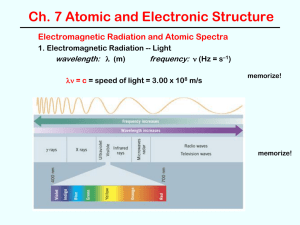
Wave Mechanics o Understand the relationship between: Wavelength, Frequency, and Energy
Solve for any of the following variables mentioned above. o Be able to describe what differentiates types of electromagnetic radiation and be able to give a detailed description on why we see certain colors. o Relate wave mechanics to the principles of the Quantum Mechanical model of atoms and how we can use our understanding of how electrons behave as an analytical tool.
Atomic Spectra (Continuous, Emission, Absorption)
**ALL OTHER TOPICS COVERED DURING CHAPTER 4 AND 5 ARE ALSO ELLIGIBILE FOR TEST MATERIAL**