Home work
advertisement
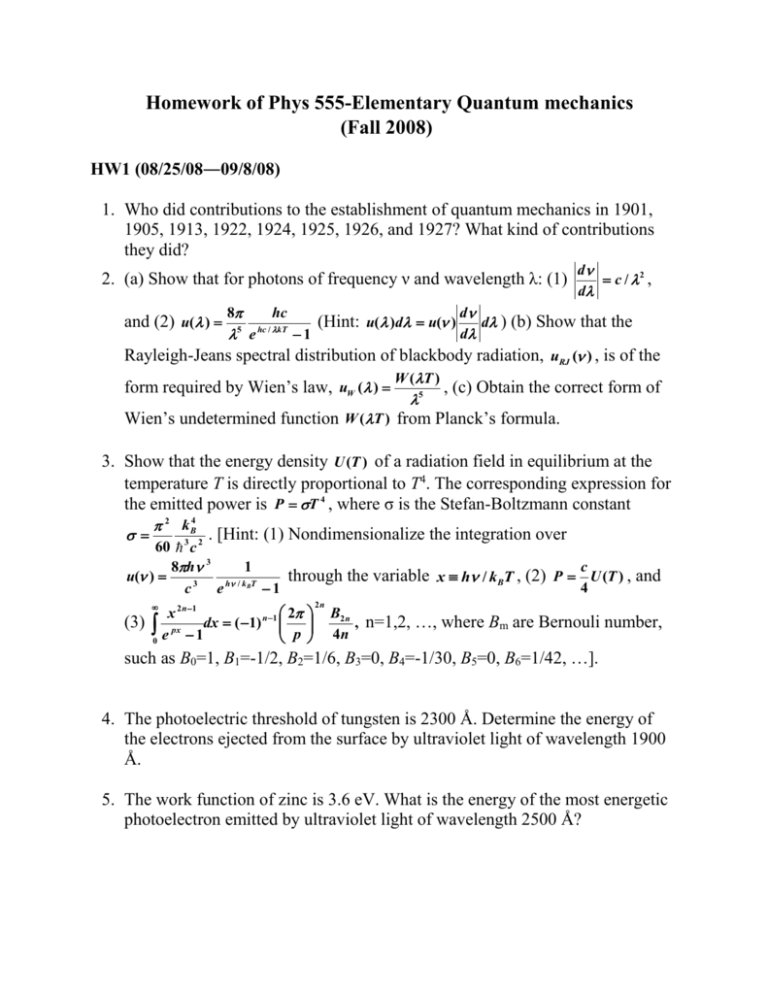
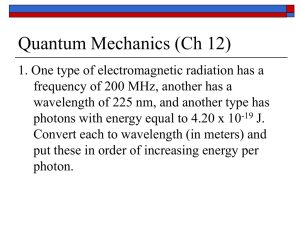
Homework of Phys 555-Elementary Quantum mechanics (Fall 2008) HW1 (08/25/08―09/8/08) 1. Who did contributions to the establishment of quantum mechanics in 1901, 1905, 1913, 1922, 1924, 1925, 1926, and 1927? What kind of contributions they did? 2. (a) Show that for photons of frequency ν and wavelength λ: (1) 8 hc 5 hc / kT d c / 2 , d d d ) (b) Show that the d e 1 Rayleigh-Jeans spectral distribution of blackbody radiation, uRJ ( ) , is of the W ( T ) form required by Wien’s law, uW ( ) , (c) Obtain the correct form of 5 and (2) u( ) (Hint: u( )d u( ) Wien’s undetermined function W (T ) from Planck’s formula. 3. Show that the energy density U (T ) of a radiation field in equilibrium at the temperature T is directly proportional to T4. The corresponding expression for the emitted power is P T 4 , where σ is the Stefan-Boltzmann constant 2 k B4 . [Hint: (1) Nondimensionalize the integration over 60 3 c 2 8h 3 1 c u( ) through the variable x h / kBT , (2) P U (T ) , and 3 h / k BT 4 c e 1 2 x 2 n 1 (3) px dx ( 1) n1 1 p 0 e 2n B2 n , n=1,2, …, where Bm are Bernouli number, 4n such as B0=1, B1=-1/2, B2=1/6, B3=0, B4=-1/30, B5=0, B6=1/42, …]. 4. The photoelectric threshold of tungsten is 2300 Å. Determine the energy of the electrons ejected from the surface by ultraviolet light of wavelength 1900 Å. 5. The work function of zinc is 3.6 eV. What is the energy of the most energetic photoelectron emitted by ultraviolet light of wavelength 2500 Å? 6. (a) What is the formula for the frequency ν of radiation emitted when the hydrogen atom decays from state n to state n ? Give your answer in terms of R, n , n , and h only. (b) What is the corresponding formula for the wavelength λ emitted in the same transition? Now your formula will contain the additional constant c, the speed of light. 7. The dimensionless number e2 4 0 c 1 is called the fine-structure 137.037 1 2 constant. (a) Show that the Rydberg constant may be written R 2mc 2 . (b) If the rest-mass energy of the electron is mc2=0.511 MeV, calculate R in eV. (c) Obtain an expression for the Bohr energies En in terms of α and mc2. 8. Show that the de Borglie wavelength of an electron of kinetic energy E(eV) is e 0.29 10 8 12.3 10 8 cm and that of proton is cm. p E 1/ 2 E 1/ 2 9. At what speed is the de Borglie wavelength of an α particle equal to that of a 10KeV photon? Homework of Phys 555-Elementary Quantum mechanics (Fall 2008) HW2 (09/12/08―09/24/08) Text book: Problem 1.1 (page 12) Problem 1.2 (page 12) Problem 1.4 (page 14) Problem 1.6 (page 18) Problem 1.8 (page 18) Problem 1.10 (page 20) Problem 1.11 (page 20) Problem 1.12 (page 21) Problem 1.15 (page 22) Problem 1.18 (page 23) Homework of Phys 555-Elementary Quantum mechanics (Fall 2008) HW3 (09/29/08―10/22/08) Text book: Problem 2.3 (page 28) Problem 2.4 (page 38) Problem 2.8 (page 40) Problem 2.9 (page 40) Problem 2.13 (page 50) Problem 2.14 (page 51) Problem 2.15 (page 57) Problem 2.18 (page 66) Problem 2.21 (page 67) Problem 2.24 (page 77) Problem 2.30 (page 83) Problem 2.31 (page 83) Homework of Phys 555-Elementary Quantum mechanics (Fall 2008) HW4 (10/29/08―11/21/08) Text book: Problem 3.2 (page 96) Problem 3.4 (page 98) Problem 3.10 (page 106) Problem 3.11 (page 109) Problem 3.13 (page 112) Problem 3.16 (page 114) Problem 3.17 (page 118) Problem 3.23 (page 124) Problem 3.24 (page 124) Homework of Phys 555-Elementary Quantum mechanics (Fall 2008) HW5 (11/17/08―12/15/08) Text book: Problem 4.1 (page 132) Problem 4.3 (page 139) Problem 4.4 (page 139) Problem 4.7 (page 144) Problem 4.8 (page 144) Problem 4.11 (page 154) Problem 4.14 (page 156) Problem 4.15 (page 156) Problem 4.21 (page 170) Problem 4.23 (page 170)