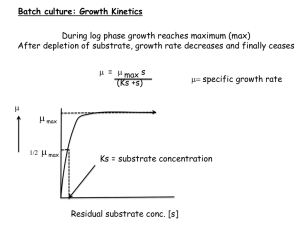
lecture 11 and 12

8-Solvent-solvent precipitation:
The extract dissolved in a suitable solvent, is mixed
with a less polar miscible solvent causing the selective
precipitation of the less soluble plant constituents.
e.g1. Precipitation of triterpenoid saponins from
methanol extract of Phytolacea dodecandra by
the addition of acetone.
9- Distillation Methods:
•
There are two types of traps:
One for oils lighter than water and the other for oils heavier than water.
• These two types differ only in the mechanism of the return of the aqueous layer to the distillation flask , keeping the volatile oil layer in its position.
Types of distillation are used:
1-Water and steam distillation
2-Direct steam distillation
Points for consideration in the distillation method:
1- It is often necessary to subject the plant material to special
treatment prior to steam distillation e.g. cut or crushed.
Crushing or cutting facilitates penetration of water into
oil- containing structures in the plant.
eg. Oil cells, glandular hairs.
2- For removal of water or moisture which might be present in
1
the prepared volatile oil, anhydrous sodium sulfate is usually
used.
Choice of solvent for extraction
1.
Be highly selective for the compound to be extracted.
2.
Have a high capacity for extraction.
3.
Not react with the extracted compound.
4.
Have a low price.
5.
Be harmless to man and to the environment.
6.
Be completely volatile.
According to the pharmacopoeias, ethanol is the solvent of choice for obtaining classic extracts.
The ethanol is usually mixed with water to induce
swelling of the plant particles and to increase the porosity of the cell walls which facilitates the diffusion of extracted substances from inside the cells to the surrounding solvent.
As a general empirical rule:
Non polar solvents (petroleum ether and hexane)
will dissolve non-polar compounds (fats and waxes).
2
While polar solvents (methanol, ethanol and water)
dissolve polar compound (alkaloid salts and sugars).
(Like dissolve like)
The affinity of the solute for the organic phase may
be greatly increased by using mixture of solvents
instead of single ones (used mixtures of solvent to
increase the solubility).
Example: solublization of an aliphatic carboxylic acid in ethanol, acetone and a mixture of both.
O------------H-O-CH -CH
3
In ethanol -R-C
Hydrogen bond
O-H
O
In acetone R-C Hydrogen bond
O-H---------O=C
CH
3
CH
3
In a mixture of acetone and ethanol
Increase solubility of carboxylic acid by addition of ethanol and acetone.
And the solubility increased due to the formation of hydrogen bonds.
O
R-C
OH
HO-C
2
H
5
(ethanol)
O=C
CH
3
CH
3
(acetone)
3
Plants remain the most important source of natural drugs, however only about 10% have been
fully studied.
- More than 30% of prescription drugs are natural products.
- More than 60% of anticancer and anti-infective drugs are natural products.
The main sources of drugs are as follows:
1- Natural
plants, microorganisms, marine plants, animals, (totally obtained from nature).
2- Semisynthetic
Such as steroidal hormones and
corticosteroids.
3- Synthetic
These are drugs which are manufactured by total synthesis
4
Why do we need to study plant metabolites?
1- The discovery of new therapeutic agents
2- The disclosing of new sources of economic materials for the synthesis of complex chemical
substances
3- Isolation of a novel chemical structure often leads the chemist to a successful synthesis of a series
of synthetic compounds which may have some medicinal value.
Plant constituents may also be known as plant metabolites
Primary and secondary plant metabolites
Plant constituents comprise a wide variety of organic substances that are formed and accumulated by plants. They include:
I-Primary metabolites which are produced by primary metabolism
The primary metabolites such as carbohydrates, proteins, fats, and nucleic acids, are essential for life and are commonly present in all organisms in large amounts.
■ Life cannot exist without primary metabolism
■ The pathways for generally modifying and synthesizing carbohydrates, proteins, fats, and nucleic acids are found to be essentially the same in all organisms, apart from minor variations.
■ These processes are collectively described as primary metabolism, with the compounds involved in the pathways being termed primary metabolites.
■ II-Secondary metabolites , also known as natural products, are those products of metabolism that are not essential for normal growth, development or reproduction of an organism. In this sense they are "secondary".
■ Each plant family, genus, and species produces a characteristic mix of these secondary
metabolites, and they can sometimes be used as taxonomic characters in classifying plants.
■ Humans use many of these compounds as medicines due to their variable biological activities.
Secondary metabolites are rare in animals, but are common in: plants, fungi and bacteria.
5
Secondary metabolites, may be produced in the plant as :
Defense against predators,
Detoxifying agents.
They include most of the pharmacologically active natural plant products. They are usually produced in small quantity in the plant.
Many secondary compounds are brightly colored pigments like anthocyanin that color flowers red and blue. These attract pollinators and fruit and seed dispersers.
Nicotine and other toxic compounds may protect the plant from herbivores and microbes.
However, some groups of natural products could be assigned to both divisions e.g. fatty acids and sugars. Most of these compounds are described as primary metabolites, whilst some representatives are of rare occurrence and characteristic to certain plant species and are thus closer to secondary metabolites.
Factors influencing the production of plant secondary metabolites
■ Heredity or genetic composition that induces both qualitative and quantitative changes.
■ Stage of development , and
■ Environmental changes that result mainly in quantitative variations.
Plant Secondary Metabolites
■ Secondary compounds are grouped into classes based on similar structures, biosynthetic pathways, or the kinds of plants that make them. The largest classes are the alkaloids, terpenoids, and phenolics.
Biogenesis of secondary plant metabolites
Biogenetic pathways
The pathways involved in the biosynthesis or biogenesis of the different types of the secondary plant constituents are dependent on the fundamental metabolic cycles of the living tissue.
These are summarized in Scheme (1).
6
Primary metabolic pathway of carbon
CO
2
O
2
Photosynthesis
Primary metabolic products
Carbohydrates
Secondary metabolic products
Complex polysaccharides,
Glycosides &
Aminoglycoside antibiotics
Sugars
Erythrose phosphate
Glycolysis
Phosphoenolpyruvate
Shikimic acid
Aromatic amino acids
Aliphatic amino acids
Proteins
Phenylpropanoids
Alkaloids, Peptides,
Penicillins &
Cephalosporins
Pyruvate
Acetyl-CoA
T ricarboxylic acid cycle
Malonyl C oA
Isoprene
Fatty acids
(lipids) Fats & Waxes
Anthraquinones,
Erythromycins &
Tetracyclines
Terpenoids
CO
2
Squalene Steroids
Scheme (1): Basic metabolic pathways and the origin of secondary metabolites
Classification of plant secondary metabolites
1-Phenolic compounds
These include a wide range of plant substances, which are recognized by their hydrophilic nature and their common origin from the aromatic precursor shikimic acid.
They possess at least one aromatic ring with one or more oxygenation sites.
7
Shikimic acid
Cinnamic acid
Ferulic acid
Caffeic acid
Flavonols
Coumarin
Isoflavone
8
Simple phenolics
Examples of plant phenolics are the flavonoids and their glycosides, the phenyl propanoids, anthocyanins, xanthones, tannins and quinones.
Many of the phenolic compounds are reported to have antioxidant and anticancer activities.
9