CHM562 Natural Products Spring 2011 Meets MWF @ 9 AM, II-307B
advertisement
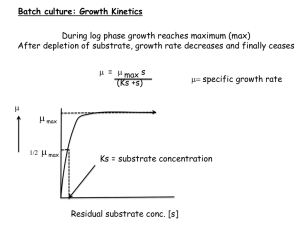
CHM562 Natural Products Meets MWF @ 9 AM, II-307B Instructor: Dr. Catherine Neto Office: II-301A Research Lab: II-301 Spring 2011 email: cneto@umassd.edu phone: x6928, or 508-910-6928 Office hours TBA _____ Pre-requisite: 1 year of Organic Chemistry & lab; biochemistry strongly recommended. Textbook: Medicinal Natural Products—a Biosynthetic Approach by Paul M. Dewick, Wiley & Sons, 2nd or 3rd edition. Not available in the bookstore, see Amazon.com: http://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Dstripbooks&fieldkeywords=Dewick%2C+Medicinal+Natural+Products&x=0&y=0 A 3-ring binder is highly recommended! Some journal articles and other course materials will be distributed; others will be available in the library or through interlibrary loan. You will also have access to recent issues of Journal of Natural Products and Journal of Agricultural and Food Chemistry through our laboratory subscription, can use the computer in II-301 to download those. 2001-2009 available in print. Notes will be posted on my faculty course webpage: http://www.faculty.umassd.edu/catherine.neto/welcome.cfm?pg=6 Course Assignments • 50% of grade based on two exams: a midterm and a final. Both will include a take home portion utilizing recent scientific literature in natural products. • 20% of grade based on course project: Write an original review article summarizing recent original research studies on a topic of your choice. • 30% of grade based on presentations and class participation. Each student will be expected to give two 15-minute presentations in class based on the scientific literature. Tentative schedule for the first part of the semester Jan. 26th- March 9th: Topics to be covered, in order of appearance: Primary & secondary metabolites: building blocks, construction mechanisms, Enzymes, vitamins & cofactors (Ch. 2 Dewick). Acetate pathway to fatty acids, polyketides, macrocyclics (Ch. 3 Dewick) Shikimate pathway to aromatic compounds & phenolics (Ch. 4 Dewick) Flavonoids and polyphenolics, antioxidant chemistry and biology (Literature) Feb 21st: President’s Day, no school Feb 22nd: It’s really Tuesday. No class. Feb 23rd to March 2nd: Student presentation #1: Info coming soon! March 11th: Exam #1 Natural Products – Where Chemistry, Botany & Pharmacology Meet! Goals of natural product research: • Generate leads for development of novel drugs • Understand structure-activity relationships • Explore medicinal uses of plants/microbes and their constituents • Study biosynthetic pathways • Scientific basis for use of foods/herbs as preventive medicine or treatment • Preserve biodiversity Primary vs. secondary metabolites…what’s the difference? • Primary metabolites: necessary for basic survival of an organism - energy and tissue construction - includes particular carbohydrates, amino acids and proteins, lipids, nucleic acids, vitamins & cofactors • Secondary metabolites: - are assembled from (pieces of) primary metabolites - may be more prevalent or unique to certain genus, species - often have vital functions in the source organism - attractants for propagation of species - defense against predators - signaling - may have useful effects on humans/other organisms Classic example of successful natural product drug: The Story of Taxol Aromatic moieties from Phe (shikimate pathway) Acetyl groups (CoA) from pyruvate, (acetate pathway) Isoprenoid ring from mevalonic acid (mevalonate pathway) Source of taxol (plant part, common name, botanical name) = Bark of Pacific yew, Taxus brevifolia (Taxaceae) Genus species (Family) • Bark collected in 1962 as part of an NCI project to find new cancer drug leads by collecting, extracting and testing plants • Extract was found active in an animal model in 1964 • The active compound was isolated and identified in 1971 Fun facts about Taxol (taxotere) • 6 trees are needed to yield enough bark for 2 g of drug used in typical course of chemo! • It took years to extract enough taxol for a clinical study • Used to treat breast, ovarian, lung cancers, Kaposi’s sarcoma and cancers of head and neck • Reduces tumor cell proliferation by binding to tubulin, disrupting formation of microtubules, blocking mitosis • Semi-synthetic derivatives can now be made starting with a less bioactive “relative” of taxol “Drugs” vs. “Botanicals” • Plants contain a diverse mixture of compounds • Extracts or powders (“botanicals”) are often more effective than any single constituent • Most contain more than one “active principle” or biologically active compound • Synergistic (additive) or complementary pharmacological effects are possible with a mixture • Side effects may be less severe with botanicals than isolated drugs, which often work precisely because they ARE toxic! • Cost of obtaining pure compounds may be high Goals for this course • Recognize structural features of natural compounds and understand where they come from..carbon skeletons, functional groups • Biosynthetic pathways: how compounds are put together – the mechanisms and the enzymes & cofactors that make it happen. • Get familiar with key classes of “secondary metabolites” and find out what the biological effects of these compounds are • Get to know some interesting plants—botanical info, historical use • Learn about methods used in natural products research: isolation, structure elucidation, biological evaluation • Learn to read and interpret pharmacological data from “bioassays” used to assess effectiveness of compounds against disease conditions • Become familiar with scientific literature in the field