Notes 4.2-4.3
advertisement

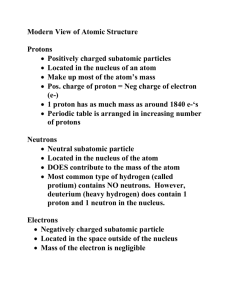
Notes 4.2 and 4.3 4.2 The Structure of the Atom Rutherford found that the nucleus is about 10,000 times smaller than the atom, and recall that electrons are only 1,840th the size of a proton or neutron..... Astonishing implications of Rutherford's evidence that the atom is mostly “empty space”: If you were to suck out all of the empty space from every atom in the human body, then it would shrink down to a size smaller than a grain of salt! If you sucked out all of the empty space from every atom in the bodies of all 7 billion humans, we would all shrink down to the size of an apple! If all matter is mainly empty space, why is it impossible to walk through walls or pass your hand through your desk? The space in an individual atom is large relative to the volume of the atom, but very small relative to an object the size of a hand. There are many layers of atoms in a wall or a desk. The space that exists is distributed evenly throughout the solid, similar to the distribution of air pockets in foam insulation. The space is not really empty, even though electrons have almost negligible mass, they have energy. If you push atoms together, the electrons repel eachother and you feel this pushing away force as “solid”. It’s like trying to force two magnets together. 4.3 Distinguishing Among Atoms A. Atomic number Elements are defined by the number of protons in the nucleus = Atomic number Examples: Hydrogen: atomic number 1 = _____proton Helium: atomic number 2 = _______ protons Copper: atomic number 29 = ______ protons Silver: atomic number 47 = _______ protons Arranged by ___________________________________ on the periodic table Number of protons = number of electrons as long as the atom is ___________________ (net charge of 0) Example: Carbon Atomic number = 6 6 protons (+6) Must have 6 electrons (-6) +6 and -6 = 0 charge 15. Complete the Table (pg. 111) Element Atomic Number Protons Electrons 16. How many protons and electrons are in each atom? a) fluorine (atomic number =9) b) calcium (atomic number = 20) c) aluminum (atomic number = 13) B. Mass number Most of the mass of an atom is in the nucleus Nucleus contains protons and neutrons, each 1840 x larger than mass of electron Mass number = ____________________________________________________ Mass number = Atomic # + # neutrons Example: Gold Shorthand notation: Mass number = 197 Atomic number = 79 # protons: 79 # electrons: # neutrons: 79 (same as protons) Mass # - atomic # 197-79 = 118 neutrons Can also be written as Gold-197 name, hyphen, mass # 17. How many neutrons are in each atom? a. b. d. e. With this information, we can determine atomic number number of protons number of electrons c. mass number number of neutrons What is another way these atoms can be written? 18. Use Table 4.2 to express the composition of each atom in shorthand form. a. carbon-12 b. fluorine-19 c. beryllium-9 C. Isotopes Def: ___________________________________________________________________________________________ Diff varieties of atoms of one element In nature, most elements occur as a _____________________________ of two or more isotopes Examples: diff isotopes of carbon are carbon-12, carbon-13, carbon-14 Isotopes must have same atomic number b/c that defines the element 6 protons makes carbon carbon Mass number is different and so isotope is identified by mass number Example of Neon isotopes All neon isotopes have atomic number of 10 Neon-20 Neon-21 Neon-22 Hydrogen 1 proton pg. 113 Practice Problems 19. Three isotopes of oxygen are oxygen-16, oxygen-17, and oxygen-18. Write the symbol for each, including the atomic number and the mass number. 20. Three isotopes of chromium are chromium-50, chromium-52, and chromium-53. How many neutrons are in each isotope, given that chromium has an atomic number of 24? Chromium-50 has _______ neutrons (50-_______ = 26) Chromium-52 has ________neutrons (52-_______ = 28) Chromium-53 has _________neutrons (53-_______ = 29) D. Atomic Mass Masses of individual atoms are inconveniently small and impractical to work with A reference isotope was chosen, carbon-12, to compare all other masses to, and assigned a mass of exactly 12 atomic mass units. atomic mass unit (amu) = ______________________________________________________________ 1 proton or 1 neutron weighs about 1 amu Helium-4 atom has a mass of 4.0026 amu, which is about 1/3 of the mass of a carbon-12 atom. Nickel-60 atom has about five times the mass of a carbon-12 atom Atomic mass = _____________________________________________________________________________ reflects both the mass and the relative abundance of isotopes as they occur in nature Table 4.3 Text pg 114 Example: chlorine-35 (amu = 34.969) accounts for 75% of the naturally occurring Cl atoms; chlorine-37 (amu= 36.966) accounts for only 25%, which gives a 3:1 ratio Text pg 115. There is more chlorine-35 than chlorine-37 in nature, so the atomic mass of chlorine is closer to 35 than to 37, at 35.5 amu. See Text pg 116 pg 116 21. Boron has two isotopes: boron-10 and boron-11. Which is more abundant, given that the atomic mass of boron is 10.81 amu? 22. There are three isotopes of silicon: they have mass numbers of 28, 29, and 30. The atomic mass of silicon is 28.086 amu. Comment on the relative abundance of these three isotopes. To calculate the atomic mass of an element, multiply the mass of each isotope by its natural abundance, expressed as a decimal, and then add the products. Mass of isotope A x Abundance of isotope A + Mass of isotope B x Abundance of isotope B *change percents to decimals before multiplying Ex: Carbon-12 has natural abundance of 98.89% Carbon-13 has a natural abundance of 1.11% (12 amu X .9889) + (13 amu X .0111) = 12.0111 amu Text pg 117 Calculating Atomic Mass - More examples 23. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for mass = 62.93 amu, and 30.8% for mass = 64.93 amu. Calculate the average atomic mass of copper. (62.93 amu x 0.692) + (64.93 amu x 0.308) = 63.546 = 63.6 amu 24. Calculate the atomic mass of bromine. The two isotopes of bromine have atomic masses and relative abundance of 78.92 amu (50.69%) and 80.92 amu (49.31%). (78.92 amu x .5069) + (80.92 x .4931) = 79.9062 = 79.91 amu A periodic table is an arrangement of elements in which the elements are separated into groups based on a set of repeating properties. A periodic table allows you to easily compare the properties of one element (or a group of elements) to another element (or group of elements). Each horizontal row of the periodic table is called a period. Within a given period, the properties of the elements vary as you move across it from element to element. Each vertical column of the periodic table is called a group, or family. Elements within a group have similar chemical and physical properties. 4.3 Section Assessment 1. What distinguishes atoms of one element from the atoms of another? 2. What equation tells you how to calculate the number of neutrons in an atom? 3. How do the isotopes of a given element differ from one another? 4. How is atomic mass calculated? 5. What makes the periodic table such a useful tool? 6. What does the number represent in the isotope platinum-194? Write the symbol for this atom using superscripts and subscripts. 7. The atomic masses of elements are generally not whole numbers. Why? 8. List the number of protons, neutrons,s and electrons in each pair of isotopes. a. Lithium-6: b. calcium-42: c. selenium-78: Lithium-7: calcium-44: selenium-80: 9. Name two elements that have properties similar to those of the element calcium (Ca). 10. Distinguish between atomic number, mass number, and atomic mass. Atomic number – Mass number- Atomic mass-