Chapter 5 and 6
advertisement
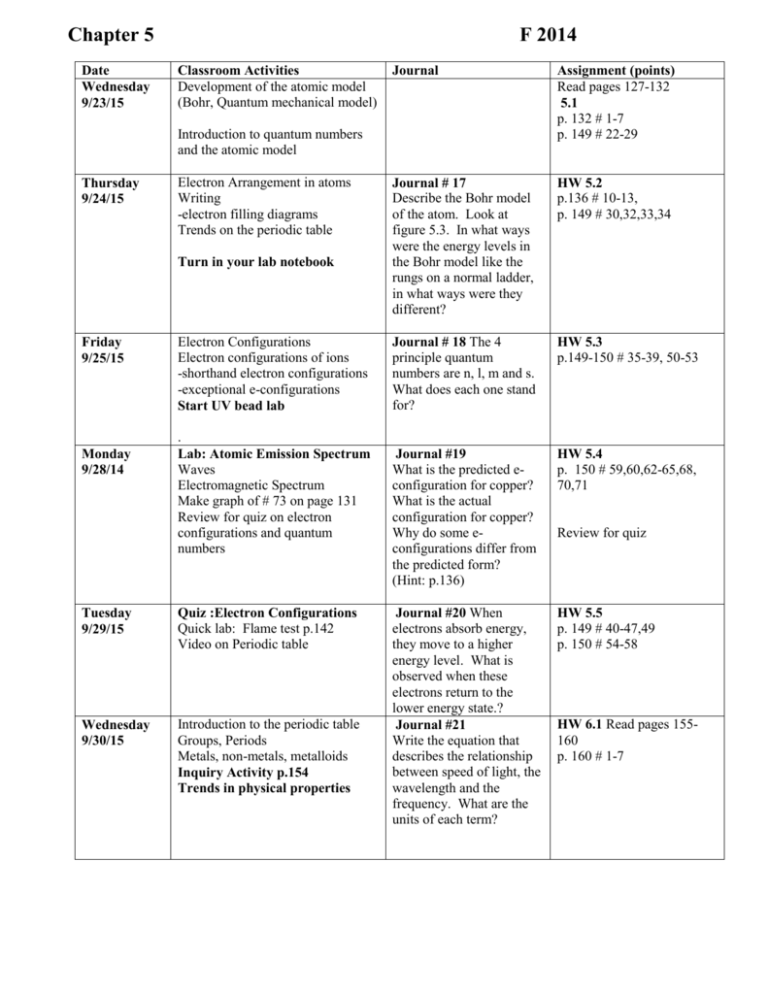
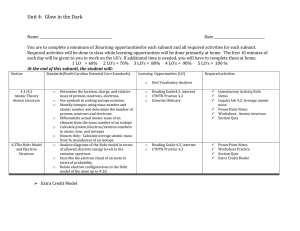
Chapter 5 Date Wednesday 9/23/15 F 2014 Classroom Activities Development of the atomic model (Bohr, Quantum mechanical model) Journal Assignment (points) Read pages 127-132 5.1 p. 132 # 1-7 p. 149 # 22-29 Journal # 17 Describe the Bohr model of the atom. Look at figure 5.3. In what ways were the energy levels in the Bohr model like the rungs on a normal ladder, in what ways were they different? HW 5.2 p.136 # 10-13, p. 149 # 30,32,33,34 Journal # 18 The 4 principle quantum numbers are n, l, m and s. What does each one stand for? HW 5.3 p.149-150 # 35-39, 50-53 Journal #19 What is the predicted econfiguration for copper? What is the actual configuration for copper? Why do some econfigurations differ from the predicted form? (Hint: p.136) HW 5.4 p. 150 # 59,60,62-65,68, 70,71 Journal #20 When electrons absorb energy, they move to a higher energy level. What is observed when these electrons return to the lower energy state.? Journal #21 Write the equation that describes the relationship between speed of light, the wavelength and the frequency. What are the units of each term? HW 5.5 p. 149 # 40-47,49 p. 150 # 54-58 Introduction to quantum numbers and the atomic model Thursday 9/24/15 Electron Arrangement in atoms Writing -electron filling diagrams Trends on the periodic table Turn in your lab notebook Friday 9/25/15 Monday 9/28/14 Electron Configurations Electron configurations of ions -shorthand electron configurations -exceptional e-configurations Start UV bead lab . Lab: Atomic Emission Spectrum Waves Electromagnetic Spectrum Make graph of # 73 on page 131 Review for quiz on electron configurations and quantum numbers Tuesday 9/29/15 Quiz :Electron Configurations Quick lab: Flame test p.142 Video on Periodic table Wednesday 9/30/15 Introduction to the periodic table Groups, Periods Metals, non-metals, metalloids Inquiry Activity p.154 Trends in physical properties Review for quiz HW 6.1 Read pages 155160 p. 160 # 1-7 Thursday 10/1/15 Lab: Alchemists Dream 2 EC points for 2 pre-1982 pennies Friday 10/2/15 Monday 10/5/15 Tuesday 10/6/15 Journal #22 What are HW 6.2 valence electrons? How many valence electrons do the Alkali metals have? How many valence electrons do the noble gases have? Read pages 161-176 Properties of other families on the periodic table Representative elements, transition elements, and noble gases Turn in lab notebooks -UV bead lab -Alchemists dream -Atomic Emission spectra -Flame test -Trends in physical properties activity Periodic Trends -Atomic Radius -Ionic Radius -atoms vs. ions Journal 23 Draw a table comparing the properties of metals, non-metals and metalloids. Include info about their location on the periodic table, appearance and conductivity. Periodic trends -Ionization Energy -Trends in Electro negativity Journal 25 Wednesday 10/7/15 Review for exam on chapter 5,6 HW check 5,6 Thursday 10/8/15 Test Ch 5,6 Lab luminescence Journal 24 Summarize the trend in atomic size as you go down a family and explain why it occurs. Explain the trend in atomic size as you go across a period. 20. What is electronegativity? What is the most electronegative metal on the periodic table? Describe the trends in electronegativity on the Periodic Table. Journal 26. Explain the trend in 1st ionization energy as you go down the alkali metals. How does this correspond to the reactivity of the metal p.181-182 # 24-35, HW 6.3 p.167 # 10-16 p. 182 # 47,48,49, 72,73,75,76 6.4 p. 178 # 16-23 p. 191 #36-46 6.5 p.182# 51,52,53,55,57,58,59,63,67, 68 Complete Review 6.6 Cumulative review p. 184 #71,73,74,75,77,78,81-85