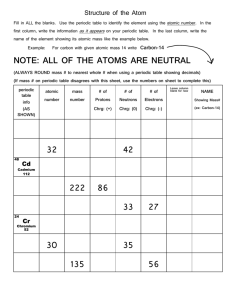
Unit 1: Matter and Energy
advertisement
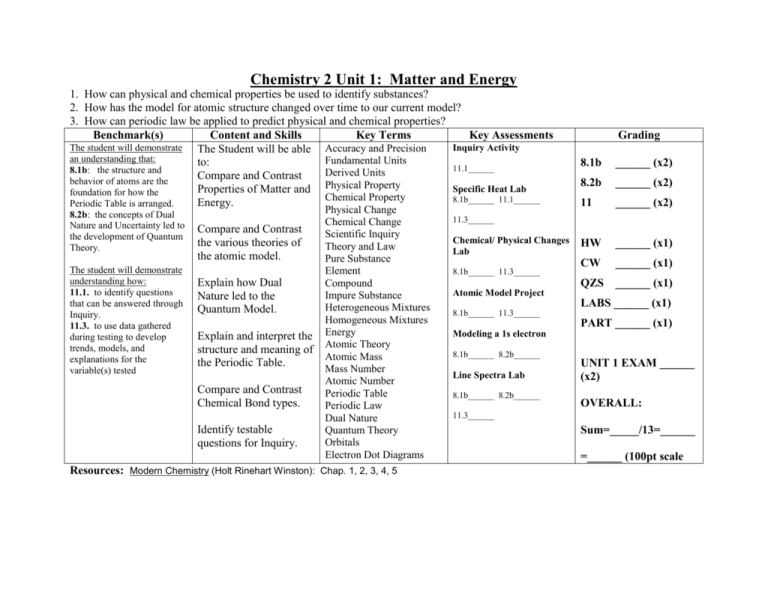
Chemistry 2 Unit 1: Matter and Energy 1. How can physical and chemical properties be used to identify substances? 2. How has the model for atomic structure changed over time to our current model? 3. How can periodic law be applied to predict physical and chemical properties? Benchmark(s) Content and Skills Key Terms Key Assessments The student will demonstrate Inquiry Activity The Student will be able Accuracy and Precision an understanding that: Fundamental Units to: 11.1______ 8.1b: the structure and Derived Units Compare and Contrast behavior of atoms are the Specific Heat Lab Properties of Matter and Physical Property foundation for how the Chemical Property 8.1b______ 11.1______ Energy. Periodic Table is arranged. 8.2b: the concepts of Dual Nature and Uncertainty led to the development of Quantum Theory. The student will demonstrate understanding how: 11.1. to identify questions that can be answered through Inquiry. 11.3. to use data gathered during testing to develop trends, models, and explanations for the variable(s) tested Compare and Contrast the various theories of the atomic model. Explain how Dual Nature led to the Quantum Model. Explain and interpret the structure and meaning of the Periodic Table. Compare and Contrast Chemical Bond types. Identify testable questions for Inquiry. Physical Change Chemical Change Scientific Inquiry Theory and Law Pure Substance Element Compound Impure Substance Heterogeneous Mixtures Homogeneous Mixtures Energy Atomic Theory Atomic Mass Mass Number Atomic Number Periodic Table Periodic Law Dual Nature Quantum Theory Orbitals Electron Dot Diagrams Resources: Modern Chemistry (Holt Rinehart Winston): Chap. 1, 2, 3, 4, 5 Grading 8.1b ______ (x2) 8.2b ______ (x2) 11 ______ (x2) HW ______ (x1) CW ______ (x1) QZS ______ (x1) 11.3______ Chemical/ Physical Changes Lab 8.1b______ 11.3______ Atomic Model Project LABS ______ (x1) 8.1b______ 11.3______ PART ______ (x1) Modeling a 1s electron 8.1b______ 8.2b______ Line Spectra Lab 8.1b______ 8.2b______ UNIT 1 EXAM ______ (x2) OVERALL: 11.3______ Sum=_____/13=______ =______ (100pt scale