Enzymes & Metabolism: Catalase Lab
advertisement

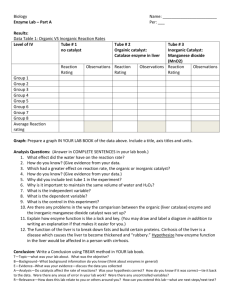
Enzymes & Metabolism Advanced Biology Enzymes are biological catalysts that carry out the thousands of chemical reactions that occur in living cells. They are large proteins made up of several hundred amino acids. In an enzyme-catalyzed reaction, the substance to be acted upon, or substrate, binds to the active site of the enzyme. The enzyme then converts the substrate to the reaction products. As is true of any catalyst, the enzyme is not used up as it carries out the reaction but is used again and again. One enzyme molecule can facilitate thousands of reactions every minute. Each enzyme is specific for a certain reaction because its amino acid sequence is unique as well as its three-dimensional structure. The active site has a specific shape so that only one or a few compounds present in the cell can interact with it. If the active site becomes blocked or changes shape the activity and efficiency of the enzyme is affected. If the changes are large enough and the enzyme can no longer act at all it is said to be denatured. Catalase (peroxidase) is one such enzyme found in living tissue. It speeds up a reaction that quickly breaks down toxic hydrogen peroxide into H2O and O2 before it can do any damage. In this lab set you’ll run both qualitative and quantitative tests for catalase activity in different tissues and under different conditions. H2O2 is a mild skin irritant, and can bleach fabric. Wear an apron, goggles, and gloves. A. Qualitative Test 1. Pour about 5ml of hydrogen peroxide solution into a clean test tube. 2. Cut off a small piece of liver; add the liver to the test tube, pushing it down with a stirring rod. Observe the reaction in the test tube, including the temperature of the test tube, and the reaction rate over time. Record your observations. 3. Light a wood splint and blow it out. While it still glows, place the burned end of the splint into the mouth of the test tube to test for oxygen gas. 4. Pour off the liquid into the sink, reserving the liver. Add fresh H2O2 to the test tube to retest the same piece of liver again. Observe and record. 5. Test for the presence of catalase in other tissues of approximately the same size. Record your observations. B. Comprehension Check 1. What type of organic molecule are enzymes? On what monomer are they built? 2. What function do enzymes serve in cells? Explain how they achieve this. 3. Why must hydrogen peroxide be metabolized by living tissues? What enzyme speeds up this reaction? 4. What are the reactants of this reaction? What is the gas produced? The liquid? 5. Use your text or other reference to look up three (3) other enzyme-substrates pairs found in living cells. 6. List four (4) variables that could affect the reaction rate. C. Quantifying O2 production Pre-Lab : In your own words, state the lab objective and summarize the following methods: 6. Using forceps, dip a filter paper disc fully into the catalase solution until soaked. Place the disc on the inside wall of a large test tube about halfway down. Repeat so you have a row of 4 discs. Pour out any excess solution that runs off the discs. 7. Measure and slowly pour 10ml of hydrogen peroxide down the inside of the test tube opposite the discs. Do not let the H2O2 come into contact with the discs. 8. Fill the plastic box half way with water. Lay the graduated cylinder on its side in the tub of water, shaking out air bubbles so that it fills completely. Turn the cylinder upside down in the water, keeping the mouth underwater at all times. Use a ring stand and clamp to hold the cylinder in place. 9. Using the stopper with tubing, run the tubing up into the graduated cylinder. With the other end, securely stopper the test tube. 10. Rotate and angle the test tube so the discs are in contact with the H2O2, yet not fully horizontal. 11. Once the discs are in the H2O2, begin timing the reaction. One group member should announce each 30 second interval, while another reads and records the ml of gas accumulated at each interval. Once during each 30 second interval, give the test tube a gentle shake to keep the reactants in contact with the peroxide. 12. Continue with the trial as long as the reaction occurs. Record both incremental and total gas produced. Graph the amount of gas produced over time. D. Quantitative lab: Adding in a variable Modify the objective and methods to reflect a variable. Write a conditional, justified hypothesis for the experiment. Run the experiment. In the previous lab we generated evidence that liver tissue contains catalase for the metabolism of hydrogen peroxide, as indicated by the bubbling off of oxygen gas, and the production of heat. In this lab, we’ll quantify the oxygen production, and then use those results as a control for an experiment. Choose from the following questions for the experiment in part B. • Is catalase found in other tissues? • Can the enzyme activity be sped up? • Does temperature affect enzyme activity? • Does pH affect enzyme activity? • Does boiling/freezing the tissue affect enzyme activity? • What is the affect of different concentrations of substrate? materials - qualitative catalase test tubes, racks, holders tissue samples - liver, cooked, and vege, muscle scales knives, cutting boards H2O2 wood splints beakers of water bunsen burner to light splint? stir rods graduated cylinder (10-25ml) safety gear quantitative catalase thermometers perioxide filter paper discs forceps grad cylinder ice water bath hot plate beaker DI water Paper towels Stopwatches 10ml cylinders tongs