Atom Spectra and Term Symbols
advertisement
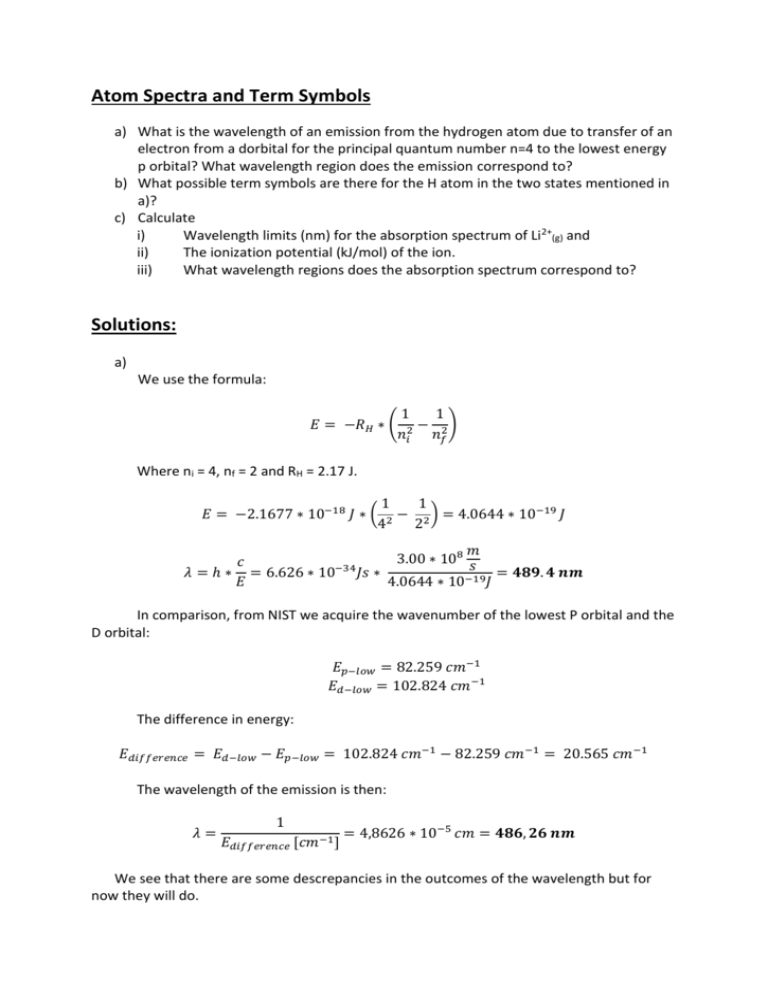
Atom Spectra and Term Symbols a) What is the wavelength of an emission from the hydrogen atom due to transfer of an electron from a dorbital for the principal quantum number n=4 to the lowest energy p orbital? What wavelength region does the emission correspond to? b) What possible term symbols are there for the H atom in the two states mentioned in a)? c) Calculate i) Wavelength limits (nm) for the absorption spectrum of Li2+(g) and ii) The ionization potential (kJ/mol) of the ion. iii) What wavelength regions does the absorption spectrum correspond to? Solutions: a) We use the formula: 1 1 𝐸 = −𝑅𝐻 ∗ ( 2 − 2 ) 𝑛𝑖 𝑛𝑓 Where ni = 4, nf = 2 and RH = 2.17 J. 1 1 𝐸 = −2.1677 ∗ 10−18 𝐽 ∗ ( 2 − 2 ) = 4.0644 ∗ 10−19 𝐽 4 2 𝑚 3.00 ∗ 108 𝑠 𝑐 𝜆 = ℎ ∗ = 6.626 ∗ 10−34 𝐽𝑠 ∗ = 𝟒𝟖𝟗. 𝟒 𝒏𝒎 𝐸 4.0644 ∗ 10−19 𝐽 In comparison, from NIST we acquire the wavenumber of the lowest P orbital and the D orbital: 𝐸𝑝−𝑙𝑜𝑤 = 82.259 𝑐𝑚−1 𝐸𝑑−𝑙𝑜𝑤 = 102.824 𝑐𝑚−1 The difference in energy: 𝐸𝑑𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒 = 𝐸𝑑−𝑙𝑜𝑤 − 𝐸𝑝−𝑙𝑜𝑤 = 102.824 𝑐𝑚−1 − 82.259 𝑐𝑚−1 = 20.565 𝑐𝑚−1 The wavelength of the emission is then: 𝜆= 1 𝐸𝑑𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒 [𝑐𝑚−1 ] = 4,8626 ∗ 10−5 𝑐𝑚 = 𝟒𝟖𝟔, 𝟐𝟔 𝒏𝒎 We see that there are some descrepancies in the outcomes of the wavelength but for now they will do. This emission belongs to the visible spectrum. b) Electron in d-orbital: We want to find the term symbols, 2s+1XJ, where s is the spin of the system and J is the rotation. Now, since only one electron resides in the d-orbital, s=1/2 : 1 2𝑠 + 1 = 2 ∗ + 1 = 𝟐 2 Furthermore, being in a d-orbital tells us that the angular momentum quantum number, l, can be: 𝑙 = −2, −1, 0, +1, +2 Hund‘s rule now tells us that we should choose the highest possible value for l, i.e. +2. l Term Symbol - X 0 S 1 P 2 D 3 F 4 G Etc. Etc. So from Hund‘s rule we find that our X is equal to D. J is given by the equation: 𝐽 = |𝐿 + 𝑠|, … , |𝐿 − 𝑠| 𝐽 = 2+ 1 1 𝟓 𝟑 ,2 − = , 2 2 𝟐 𝟐 So we can now round up the possible term symbols for the d-orbital. 2 𝐷5 , 2𝐷3 2 2 Using these exact steps, we find the term symbols for the p-orbital to be: 2 𝑃3 , 2𝑃1 2 2 c) i) Since Li2+ only has one electron we can expand Rydberg‘s formula, which was used in a). 1 1 𝐸𝑙𝑜𝑤 = −𝑅𝐻 ∗ 𝑍 2 ∗ ( 2 − 2 ) , 𝑤ℎ𝑒𝑟𝑒 𝑍 𝑖𝑠 𝑡ℎ𝑒 𝑛𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑝𝑟𝑜𝑡𝑜𝑛𝑠 𝑖𝑛 𝑡ℎ𝑒 𝑛𝑢𝑐𝑙𝑒𝑢𝑠 𝑛𝑖 𝑛𝑓 1 1 𝐸𝑙𝑜𝑤 = −2,1677 ∗ 10−18 𝐽 ∗ 32 ∗ ( 2 − 2 ) = 1.4632 ∗ 10−17 𝐽 2 1 𝜆𝑙𝑜𝑤 𝑚 3.00 ∗ 108 𝑠 𝑐 −34 = ℎ ∗ = 6.626 ∗ 10 𝐽𝑠 ∗ = 13.6 𝑛𝑚 𝐸 1.4632 ∗ 10−17 𝐽 𝐸ℎ𝑖𝑔ℎ = −2,1677 ∗ 10−18 𝐽 ∗ 32 ∗ ( −34 𝜆ℎ𝑖𝑔ℎ = 6.626 ∗ 10 1 1 − 2 ) = 1.9509 ∗ 10−17 𝐽 2 ∞ 1 𝑚 3.00 ∗ 108 𝑠 𝐽𝑠 ∗ = 10.2 𝑛𝑚 1.9509 ∗ 10−17 𝐽 NIST however gives us: 𝐸𝑙𝑜𝑤 = 476035 𝑐𝑚−1 → 𝜆𝑙𝑜𝑤 = 21.0 𝑛𝑚 𝐸ℎ𝑖𝑔ℎ = 605691 𝑐𝑚−1 → 𝜆ℎ𝑖𝑔ℎ = 16.5 𝑛𝑚 So again we see that the Rydberg formula gives us a relatively crude assessment of the energies of emission for hydrogen-like atoms. ii) The ionization potential is simply the maximum absorbance possible for the Li2+ atom (given we use the Rydberg formula), when n ∞. 𝐼. 𝑃. = 1.9509 ∗ 10−17 𝐽 = 11,748 𝑘𝐽 𝑚ó𝑙 NIST however gives the ionization potential: 𝐼. 𝑃. = 610079 𝑐𝑚−1 = 7298.16 𝑘𝐽 𝑚ó𝑙 iii)The emission spectra for Li2+ is in the UV-spectrum but very close to the X-Ray limit (~10 nm). Höfundur úrlausnar: Helgi Rafn Hróðmarsson, október, 2010