Ch 25 and 5 Practice Test KEY
advertisement

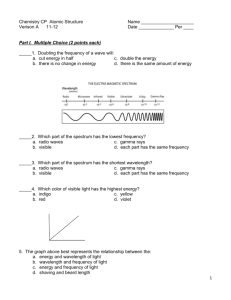
Chemistry CP Atomic Structure Verison A 11-12 Name _____________________ Date ______________ Per ____ Part I. Multiple Choice (2 points each) _____1. Doubling the frequency of a wave will: a. cut energy in half c. double the energy b. there is no change in energy d. there is the same amount of energy _____2. Which part of the spectrum has the lowest frequency? a. radio waves c. gamma rays b. visible d. each part has the same frequency _____3. Which part of the spectrum has the shortest wavelength? a. radio waves c. gamma rays b. visible d. each part has the same frequency _____4. Which color of visible light has the highest energy? a. indigo c. yellow b. red d. violet 5. The graph above best represents the relationship between the: a. energy and wavelength of light b. wavelength and frequency of light c. energy and frequency of light d. shaving and beard length 1 Use the diagram below to answer the following questions: _____ 1s2 _____ 2s2 _____ _____ _____ 2p6 _____ 3s2 _____ 6. What does the “2” in 2p6 represent? a. energy level b. sublevel _____ _____ _____ 3p1 c. orbital d. # of electrons _____ 7. What does the “6” in 2p6 represent? a. energy level c. orbital b. sublevel d. # of electrons _____ 8. What do the lines represent? a. energy levels b. sublevels c. orbitals d. # of electrons _____ 9. Which element is represented by the orbital diagram? a. sulfur c. aluminum b. phosphorus d. boron _____ 10. The natural nuclear emission with the greatest penetrating power is a. alpha particles c. gamma rays b. Beta particles d. positron emission _____ 11. The natural nuclear emission that will be attracted toward a positive electric field is a. alpha particles c. gamma rays b. Beta particles d. positron emission ______ 12. Which nuclear emission represents the greatest loss in mass: a. alpha radiation c. beta decay b. gamma radiation d. positron emission 2 PART II: SHORT ANSWER: 1 POINT EACH 3 2 A C 1 B D Use the diagram above to answer the following questions: (circle the correct answer) 10. Which letters represent jumps that require energy to be absorbed? A&C 11. Which number represents the ground state of an electron of hydrogen? 12. Which number represents the highest excited state? 1 2 1 2 B&D 3 3 Wavelength amplitude 13. On the diagram above label the wavelength. 14. On the diagram above label the amplitude. 15. On the diagram to the left, draw an arrow that points to the hottest part of the flame. 3 16. Write the complete reaction for each nuclear process below: (2 points each) a. 210 87𝐹𝑟 (alpha decay ) b. 42 18𝐴𝑟 (beta decay with gamma radiation) d. 41 20𝐶𝑎 (beta capture) 4 2𝐻𝑒 + 206 85𝐴𝑡 42 0 18𝐴𝑟 −1𝑒 + 42 19𝐾 210 87𝐹𝑟 41 0 20𝐶𝑎 + −1𝑒 +γ 41 19𝐾 17. Fill in the blanks to complete the reactions below: (1 point each) a. 12 _ 7𝑁_____ + b. 6 3𝐿𝑖 1 + 0𝑛 1 12 0𝑛 6𝐶 + 1 1𝐻 3 _ 1𝐻 _____ + 4 2𝐻𝑒 18. The half life of Ac-228 is 6.13 hours. unreactive after 24.52 hours? (3 points) Half life 0 1 2 3 4 Time 0 6.13 hrs 12.26 hrs 18.39 hrs 24.52 hrs mass 540g 270g 135g 67.5g 33.75g OR How much of a 540g sample of Ac-228 remains 540g 270g 135g 67.5g 33.75g 6.13hr 12.26hr 18.39hr 24.52hr 33.75g 19. Determine the half-life of an isotope if a 840g sample of the isotope decays to 52.5g in 625 days. (3 points) Half life 0 1 2 3 4 Time mass ?? 840g ?? 420g ?? 210g ?? 105g ?? 52.5g 625 days = 156.25 days 4 half lives OR 840g 420g 210g 105g 52.5g 1 2 3 4 625 days = 156.25 days 4 half lives 156.25 days 4 Fill in the chart below: You may use the abbreviated electron configuration and abbreviated orbital filling diagram. Electron Configuration (2 points each) Element Orbital Filling Diagram (2 points each) 11Na 1s22s22p63s2 56Ba [Xe] 6s2 [Xe] _____ 6s 27Co [Ar] 4s23d7 [Ar] ___ ___ ___ ___ ___ ___ 4s 3d 40Zr [Kr] 5s24d2 [Kr] ___ ___ ___ ___ ___ ___ 5s 4d Electron Dot Diagram (1 pt each) ___ ___ ___ ___ ___ ___ 1s 2s 2p 3s 5 6