FLAME TEST AND ATOMIC SPECTRA LAB
advertisement
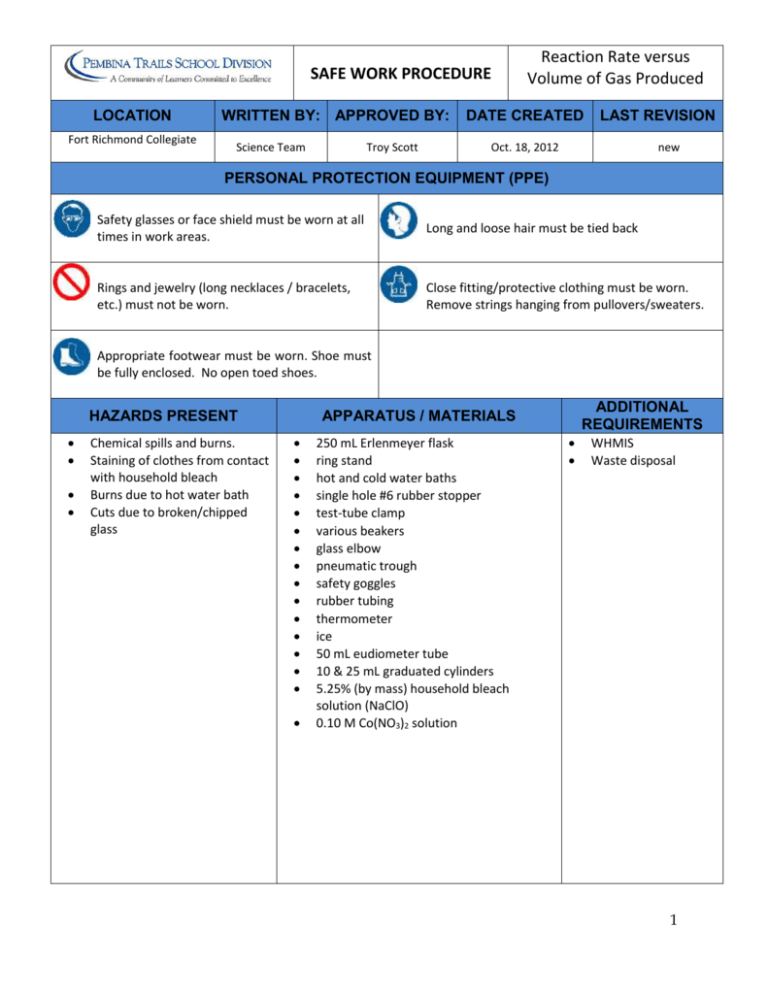
SAFE WORK PROCEDURE LOCATION Fort Richmond Collegiate WRITTEN BY: APPROVED BY: Science Team Troy Scott Reaction Rate versus Volume of Gas Produced DATE CREATED LAST REVISION Oct. 18, 2012 new PERSONAL PROTECTION EQUIPMENT (PPE) Safety glasses or face shield must be worn at all times in work areas. Long and loose hair must be tied back Rings and jewelry (long necklaces / bracelets, etc.) must not be worn. Close fitting/protective clothing must be worn. Remove strings hanging from pullovers/sweaters. Appropriate footwear must be worn. Shoe must be fully enclosed. No open toed shoes. HAZARDS PRESENT Chemical spills and burns. Staining of clothes from contact with household bleach Burns due to hot water bath Cuts due to broken/chipped glass ADDITIONAL REQUIREMENTS APPARATUS / MATERIALS 250 mL Erlenmeyer flask ring stand hot and cold water baths single hole #6 rubber stopper test-tube clamp various beakers glass elbow pneumatic trough safety goggles rubber tubing thermometer ice 50 mL eudiometer tube 10 & 25 mL graduated cylinders 5.25% (by mass) household bleach solution (NaClO) 0.10 M Co(NO3)2 solution WHMIS Waste disposal 1 SAFE WORK PROCEDURE Apparatus Set Up: Procedure: 1. Put on safety goggles and set up the apparatus as shown. 2. Ensure that the eudiometer is completely filled with water and inverted into the trough (trough should be about half-filled with water). 3. Place the loose end of the rubber tubing into the neck of the eudiometer. 4. Very carefully measure 20.0 mL of the room temperature bleach solution into the 25 mL graduated cylinder and pour it into the Erlenmeyer flask. If any spills occur, wipe them up immediately. Wash hands if bleach touched skin. 5. Record room temperature. 6. Carefully measure 7.00 mL of the room temperature cobalt (II) nitrate solution in the 10 mL graduated cylinder. 7. When all is ready, quickly pour the cobalt nitrate solution into the Erlenmeyer flask (avoid splashing), immediately place the stopper/glass elbow/rubber tube assembly on the flask, and record the time of mixing as 0 minutes. 8. You must gently, but continually, swirl the contents of the flask in order that the gas release will be consistent. Keep the swirling effect consistent! 9. Record the total volume of O2 collected at half-minute (30s) intervals until a volume of 50.0 mL is reached. Also record the actual elapsed time when the 50 mL mark is reached. WASTE DISPOSAL: After each trial, pour the contents of the Erlenmeyer flask into a large waste beaker. Later, this beaker can be added to the larger waste container provided at the front of the classroom. 10. Repeat the experiment for each of the new conditions listed below: (in all cases record the total volume of O2 collected as before.) a) Use the same quantities but place the Erlenmeyer flask and contents and the 10 mL graduated cylinder and contents in a hot water bath until the temperature of the contents are about 12oC higher than room temperature. (Then mix and collect). Water bath is hot, be careful to avoid burns from the hot plate, beaker, and water/steam). b) Repeat as per part a) except use an ice bath until the temperature of the contents are about 12oC cooler than room temperature. c) Return to the room temperature approach, but add 20.0 mL of water to the bleach solution before mixing. (Be sure to avoid splashing when combining) d) Repeat as per c), but add 40.0 mL of water to the bleach solution before mixing. e) Optional: If you have time, repeat the very first trial, but use half as much catalyst (3.5 mL instead of 7 mL). 2 REGULATORY REQUIREMENTS WS&H Act W210, Section 4, 5 Mb. Regulations 217/2006, Part 16, (Machines / Tools & Robots) Sections 16.1-16.18) Part 35, (WHMIS Application) Part 36, (Chemical & Biological Substances Application) Reaction Kinetics: Reaction Rate vs. Volume of Gas Produced Name: _________________________ Lab Partners: ________________________________ The previous experiment demonstrated that you were able to track reaction rate using indirect product mass determination. In addition, reaction rates for certain instants and intervals were calculated using primarily graphical methods. Of course, mass determinations are not the only method of rate determination. This information is obtainable in a variety of ways. For instance, if a gas is produced in a closed container, then continuous monitoring of the pressure indicates the rate. If a colour is produced or used up, monitoring of the colour intensity with a spectrophotometer (colorimeter) indicates the rate. Yet another method of monitoring the rate of a reaction involving gases is to measure the volume of gas produced by displacing water from a eudiometer (gas measuring tube). It is this latter method that will be used in this experiment. Ordinary household bleach is an aqueous solution of sodium hypochlorite, NaClO, containing a little more than 5.0% NaClO by mass. The bleaching action is caused by the hypochlorite ion (ClO-). Under normal circumstances, the hypochlorite ion breaks down slowly to give oxygen gas and the chloride ion. Equation 1: 2ClO-(aq) → 2Cl-(aq) + O2(aq) To speed this reaction to a measurable rate, a catalyst is required. In this experiment, the catalyst is provided by the addition of cobalt (II) nitrate solution to the bleach. A black precipitate of cobalt (III) oxide forms and acts as the catalyst for the decomposition of ClO-. The volume of oxygen produced is measured at 30 s intervals by displacing water from a eudiometer. From the results, you can calculate the average rate of oxygen evolution in milliliters per unit time. The experiment is repeated at other temperatures and concentrations of ClO- and the effect of these changes on the rate is observed. Purpose: 1. To practice the art of rate determination using volume of gas produced. 2. To determine the effect of temperature and concentration on reaction rate. Apparatus and Materials: See SWP above Procedure: See SWP above Results and Analysis: Results: Report, in some organized fashion, all of the raw data collected by your lab group. 3 Analysis: 1) Determine the average rate of reaction for the collection of 50.0 mL for each of the experimental levels. That should give you 5 (or 6 if you did the optional trial) average rate values. For simplicity, call them trials #1-6 in order given within the method section. Show all calculations. 2) Each group will report their average reaction rates on the board. Copy these in your journal in an organized way. 3) Analyze the class results closely. Use appropriate logic, % difference analysis, etc. It may be helpful to compare some of the more extreme average rates to the average of the average rates in a % difference manner. Discard any trials which are suspect and recalculate averages. Make some analytical statements but do not summarize/conclude. Summary Statements: For each effect (temperature, concentration, and catalyst quantity), write a short paragraph which summarizes the relationships that apparently exists between reaction rate and that effect. Give an indication of the level of confidence you have in your comments/conclusions. Include typical sources of error for each. Post Lab Considerations: As mentioned earlier, the reaction shown below is slow under normal uncatalyzed conditions. Equation 1: 2ClO-(aq) → 2Cl-(aq) + O2(aq) Often we represent catalyzed reactions by simply placing the chemical formula of the catalyst above the equation: Co(NO3)2 Equation 2: 2ClO-(aq) → 2Cl-(aq) + O2(aq) This, however, falls short of explaining how this reaction actually occurs. In this case, it would likely be more accurate to express the way this reaction occurs as follows: Step #1: Co2+ reacts with ClO12Co2+(aq) + ClO1-(aq) + 2H2O(l) → Co2O3(s) + 4H1+(aq) + Cl1-(aq) This step actually generates the active catalyst (Co2O3). Step #2: Catalytic production of O2: Co2O3(s) 2ClO-(aq) → 2Cl-(aq) + O2(aq) 4 Questions: 1. Is this a case of heterogeneous or homogeneous catalysis? (explain) 2. Can you suggest a good, creative, practical use for the principle reaction studied during this activity? 3. Increased temperature often increases reaction rate of catalyzed biochemical reactions up to some point – after which the rate may drop off. Can you think of a reason for this? (explain). 4. Within common bleach solution, there is an equilibrium condition as indicated in the equation below. Cl2(g) + 2OH-(aq) ↔ Cl-(aq) + ClO1-(aq) + H2O(l) If an unsuspecting bleach consumer mixes bleach with another cleaner or household product that contains an acid, the above equilibrium reaction may proceed aggressively toward the left (i.e. the reverse reaction rate can become very fast). Can you see the danger in this? What would happen? (explain) 5. Consider the possibility of using commercial bleach with 10% sodium hypochlorite. Sketch one graph of Total Volume O2 collected vs. time and show two results on it as follows (in a comparative fashion only): i) 1st trial with 5.25% bleach and ii) an identical trial but with 10% bleach These are sketches only, but be sure to show the shape of each graphed relationship and give a good indication of the likely difference in position between the two. Evaluation: Raw group data....10 Analysis step 1......6 Analysis step 2.....10 Analysis step 3....6 Summary...........10 Questions........8 Total..............50 5