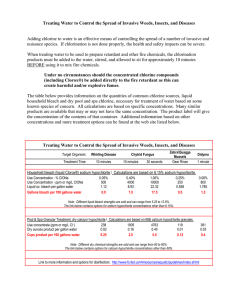
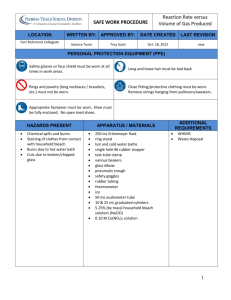
Something with A Twist
advertisement

Solution Stoichiometry: Something with a Twist 1. During the developing process of black and white film, silver bromide is removed from photographic film by the fixer. The major component of the fixer is sodium thiosulfate. The net ionic equation for the reaction is: AgBr + 2 S2O32Ag(S2O3)3- + Br- . What mass of AgBr can be dissolved by 1.00L of 0.200M Na2S2O3? 2. Calculate the volume of a 0.156M CuSO4 solution that would react with 7.89g of zinc. Hint: Write a balanced equation for this single replacement reaction. 3. A 6.77g sample of a mixture was analyzed for barium ions by adding a small excess of sulfuric acid to an aqueous sample. The resultant reaction produced a precipitate of barium sulfate which was collected by filtration, wash, dried and weighed. If 0.4123g of barium sulfate was obtained, what was the mass percent of barium in the sample? Hint: Write the net ionic equations for the precipitate reaction. 4. A useful application of oxalic acid is the removal of rust, Fe2O3, from bathtub rings according to the reaction: Fe2O3 + 6 H2C2O4 2 Fe(C2O4)33- + 3 H2O + 6 H+ Calculate the number of grams of rust that can be removed by 500ml of a 0.100M solution of oxalic acid. 5. If 100.0ml of a 0.25M AgNO3 is reacted with 125ml of 0.15M KBr, how many grams of AgBr will form? 6. If you want a 150ml of a 5.0M solution of HBr, how would you prepare the solution from a HBr solution of 12.0M 7. Laws passed in some states define a drunk driver as one who drives with a blood alcohol level of 0.10% by mass or higher. The level of alcohol can be determined by titrating blood plasma with potassium dichromate according to the equation: 16 H+ + 2 Cr2O72- + C2H5OH 4 Cr3+ + 2 CO2 + 14 H2O Assuming that the only substance that reacts with the dichromate in blood plasma is alcohol, is a person legally drunk if 38.49ml of 0.723M potassium dichromate is required to titrate a 50.0 gram sample of blood plasma? 8. Laundry bleach is a solution of sodium hypochlorite. To determine the hypochlorite, ClO-, content of bleach, sulfide ion is added in basic solution. The balanced equation for the reaction is: ClO- + S2- + H2O Cl- + S + 2OHThe chloride ion resulting from the reduction of HClO is precipitated as AgCl. When 50.0ml of laundry bleach (d=1.02g/ml) is treated as described above, 4.95g of AgCl is obtained. What is the mass percent of NaClO in bleach?