docx - STAO
advertisement

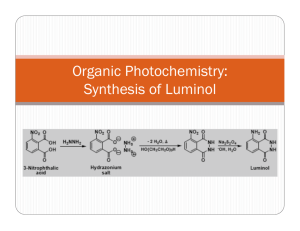
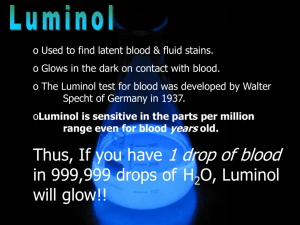
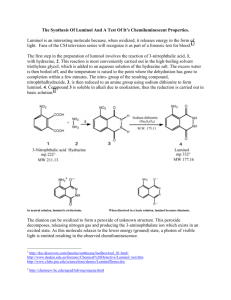
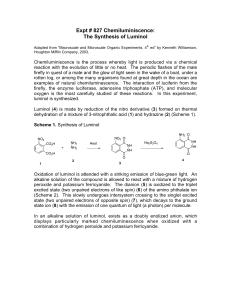
SNC2D/2P Chemical Reactions; Light and Geometric Optics/Chemical Reactions and their Practical Applications Teacher Demo: Chemiluminescence Topics Timing evidence of chemical reactions or types of light preparation: 10 min emissions demonstration: 10–15 min Specific Expectations SNC2D A1.10 draw conclusions based on inquiry results and research findings, and justify their conclusions C3.3 describe the types of evidence that indicate chemical change (e.g., changes in colour, the production of a gas, the formation of a precipitate, the production or absorption of heat, the production of light) E3.1 describe and explain various types of light emissions (e.g., chemiluminescence, bioluminescence, incandescence, fluorescence, phosphorescence, triboluminescence; from an electric discharge or light-emitting diode [LED]) SNC2P A1.10 draw conclusions based on inquiry results and research findings, and justify their conclusions E3.1 describe various types of light emissions (e.g., chemiluminescence, bioluminescence, incandescence, electric discharge) and how they produce light Introduction This demonstration is definitely an eye-catching crowd-pleaser. It is a great demonstration to show the interconnection between physics and chemistry, linking an example of light emission to evidence of chemical reactions. It is similar to a common example of bioluminescence, which occurs in fireflies, allowing them to “glow” in the dark. Materials chemical safety goggles lab coat or apron protective gloves 0.05 g luminol 100 mL 1.0 mol/L sodium hydroxide solution, NaOH(aq) 10 mL bleach 90 mL distilled water ice bath electronic balance (± 0.01 g) 100 mL graduated cylinder two 250 mL beakers round-bottomed flask or Erlenmeyer flask (at least 400 mL) Safety Considerations Provide MSDS sheets for all chemicals used. Sodium hydroxide and bleach are corrosive and toxic. Avoid skin or eye contact. Safety goggles, protective gloves, and a lab coat or apron should be worn when handling these solutions. Wash your hands and flush your eyes immediately if you come into contact with either substance. Luminol is a combustible solid. Keep it away from any heat sources and open flames. Luminol is slightly toxic by ingestion. If ingested do not induce vomiting and call poison control immediately. If luminol comes into contact with your eyes, flush them immediately with water or eye-wash fluid. Hazardous Materials Identification System Rating (0-minimal 1-slight 2-moderate 3-serious 4-severe) 1.0 mol/L sodium hydroxide luminol solution commercial bleach (5% NaClO(aq)) Procedure Wear appropriate PPE: chemical safety goggles, lab coat or apron, protective gloves. For best results, prepare the following solutions at least 15 min before performing the demonstration. 1. Prepare an ice bath large enough for two 250 mL beakers. 2. In the first beaker, dissolve 0.05 g luminol in 100 mL of 1.0 mol/L sodium hydroxide solution, NaOH(aq). 3. In the second beaker, prepare a 0.5 % sodium hypochlorite solution, NaClO(aq), by adding 10 mL of commercial bleach to approximately 90 mL distilled water. 4. Place both solutions in the ice bath until chilled (15–30 min). During class: 5. Turn off the lights for dramatic effect. 6. Observe Instruct students to watch carefully as you slowly pour the two solutions in the roundbottomed or Erlenmeyer flask. Swirl to mix the solutions. 7. Explain Have students explain what they observed. Disposal Add vinegar to neutralize the final mixture prior to disposal. Follow disposal procedures that are consistent with school board protocol and appropriate for your municipality. What happens? When the solutions are mixed, a blue glow will result. The solution will be a yellow/green colour when the lights are turned back on. How does it work? Luminol (2-aminophthalhydrazide) is an organic compound that is colourless and unremarkable in a neutral or acidic solutions, but emits bright blue light when it undergoes oxidation under basic conditions. This is an example of a chemiluminescent reaction, which is a reaction which produces light (and usually a small amount of heat). The simplified chemical equation below does not include all of the steps in this reaction, one of which involves oxygen, to produce the anion shown below. colourless yellow/green; blue light emitted Teaching Suggestions/Hints 1. 2. Prepare the solutions 15 min prior to performing the demonstration. For added dramatic effect, you could pour both solutions simultaneously into a glass or plastic spiral tube down into a beaker or flask. Hydrogen peroxide solution, H2O2(aq) and potassium ferricyanide, K3[Fe(CN)6], can be used instead of bleach. See additional resources for link to instructions. Next Steps Students could investigate chemical reactions that produce other forms of energy such as electricity as well as heat and light. Students could research bioluminescence and how it is used by some bacteria, phytoplankton, and animals. Additional Resources 1. 2. 3. Video of demonstration performed very creatively: http://www.mindbites.com/lesson/4875-chemistry-demo-luminol Detailed instructions of the chemistry of the reaction as well as demonstration instructions using hydrogen peroxide and potassium ferricyanide instead of bleach: http://www.carolina.com/category/teacher+resources/classroom+activities/luminol+the+gl owing+reaction.do Chemical reaction retrieved from: http://ncsu.edu/project/chemistrydemos/Light/Luminol.pdf